CDH2-microRNA Axis Provides Insights into Sustained Breast Cancer Dormancy in the Bone Marrow Niche
DOI:
https://doi.org/10.13052/ijts2246-8765.2024.001Keywords:
N-cadherin, breast cancer, cancer stem cell, dormancy, bone marrowAbstract
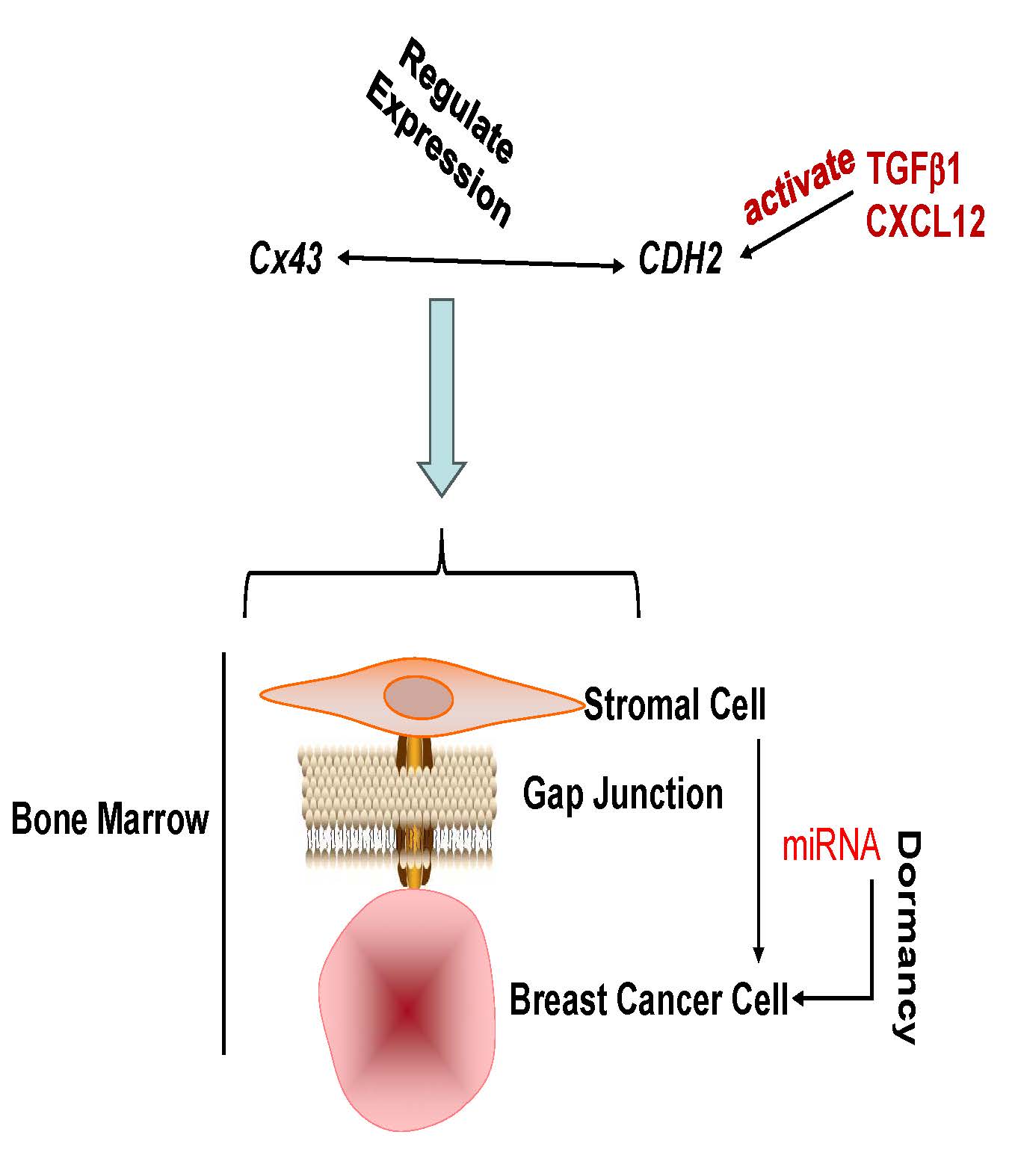
Despite long-term remission of breast cancer (BC), cancer resurgence remains a clinical issue. This issue is mostly due to BC cells (BCCs) being able to survive in dormancy for decades. This is particularly relevant to the bone marrow (BM) where the dormant BCCs survive as cancer stem cells (CSCs). The BM niche maintains BCCs in dormancy, and also mediates dedifferentiation of BCCs to CSCs. Since dormancy can occur at any time during the disease, including decades before clinical diagnosis, it is important to understand how these cells survive to allow for the development of safe treatments. Dormant BCCs establish gap junctional intercellular communication (GJIC) with BM niche such as fibroblasts and mesenchymal stem cells (MSCs). GJIC requires connexin 43 (Cx43) but cannot be a safe target since this connexin is also important for hematopoietic function. This study focused on N-cadherin (CDH2) because it facilitates Cx43-mediated GJIC between BCCs and BM niche cells. We found Cx43 and CDH2 mutually regulated their gene expression. Cloning of the 5′ regulatory regions of CDH2 unraveled DNA sequences as possible elements of repressor and activator transcription. We identified potential transcription factors (TFs) that could regulate CDH2 and showed how miRNAs that could cross GJIC might be responsible for regulating the TFs. The axis developed by CDH2 and miRNAs provide insights into how CDH2 is maintained at low level to sustain BC dormancy. The findings, together with previous findings, provide avenues for therapeutic intervention to safely reverse and target dormant BCCs in the BM niche.
Downloads
References
Siegel, R.L., K.D. Miller, and A. Jemal, Cancer statistics, 2020. CA Cancer J Clin, 2020. 70(1): p. 7–30.
Minn, A.J., et al., Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest, 2005. 115(1): p. 44–55.
Patel, S.A., et al., Treg/Th17 polarization by distinct subsets of breast cancer cells is dictated by the interaction with mesenchymal stem cells. J Cancer Stem Cell Res, 2014. 2014(2).
Lim, P.K., et al., Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res, 2011. 71(5): p. 1550–60.
Moharita, A.L., et al., SDF-1alpha regulation in breast cancer cells contacting bone marrow stroma is critical for normal hematopoiesis. Blood, 2006. 108(10): p. 3245–52.
Moore, C.A., et al., A 3D Bioprinted Material That Recapitulates the Perivascular Bone Marrow Structure for Sustained Hematopoietic and Cancer Models. Polymers (Basel), 2021. 13(4).
Sinha, G., et al., Specific N-cadherin-dependent pathways drive human breast cancer dormancy in bone marrow. Life Sci Alliance, 2021. 4(7).
Demicheli, R., M. Terenziani, and G. Bonadonna, Estimate of tumor growth time for breast cancer local recurrences: rapid growth after wake-up? Breast Cancer Res Treat, 1998. 51(2): p. 133–7.
Mansi, J.L., et al., The fate of bone marrow micrometastases in patients with primary breast cancer. J Clin Oncol, 1989. 7(4): p. 445–9.
Patel, S.A., et al., Delineation of breast cancer cell hierarchy identifies the subset responsible for dormancy. Sci Rep, 2012. 2: p. 906.
Mansi, J., et al., Bone marrow micrometastases in early breast cancer-30-year outcome. Br J Cancer, 2016. 114(3): p. 243–7.
Dawood, S., L. Austin, and M. Cristofanilli, Cancer Stem Cells: Implications for Cancer Therapy. Oncology (Williston Park), 2014. 28(12).
Rahim, F., et al., Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res, 2014. 2014: p. 405920.
Malladi, S., et al., Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell, 2016. 165(1): p. 45–60.
Correction: Cytokeratin-Positive Cells in the Bone Marrow and Survival of Patients with Stage I, II, or III Breast Cancer. N Engl J Med, 2000. 343(4): p. 308.
Braun, S., et al., Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol, 2000. 18(1): p. 80–6.
Habeck, M., Bone-marrow analysis predicts breast-cancer recurrence. Mol Med Today, 2000. 6(7): p. 256–7.
Corcoran, K.E., et al., Mesenchymal Stem Cells in Early Entry of Breast Cancer into Bone Marrow. PLOS ONE, 2008. 3(6): p. e2563.
Sandiford, O.A., et al., Mesenchymal Stem Cell-Secreted Extracellular Vesicles Instruct Stepwise Dedifferentiation of Breast Cancer Cells into Dormancy at the Bone Marrow Perivascular Region. Cancer Res, 2021. 81(6): p. 1567–1582.
Bliss, S.A., et al., Mesenchymal Stem Cell-Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res, 2016. 76(19): p. 5832–5844.
Onitilo, A.A., et al., Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res, 2009. 7(1–2): p. 4–13.
Matsuda, T., et al., N-cadherin signals through Rac1 determine the localization of connexin 43 in cardiac myocytes. J Mol Cell Cardiol, 2006. 40(4): p. 495–502.
Taniguchi Ishikawa, E., et al., Connexin-43 prevents hematopoietic stem cell senescence through transfer of reactive oxygen species to bone marrow stromal cells. Proc Natl Acad Sci U S A, 2012. 109(23): p. 9071–6.
MacNamara, K.C., et al., Transient activation of hematopoietic stem and progenitor cells by IFNgamma during acute bacterial infection. PLoS One, 2011. 6(12): p. e28669.
Reya, T., et al., Stem cells, cancer, and cancer stem cells. Nature, 2001. 414(6859): p. 105–11.
Wei, C.J., et al., Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem, 2005. 280(20): p. 19925–36.
Soh, B.S., et al., N-cadherin prevents the premature differentiation of anterior heart field progenitors in the pharyngeal mesodermal microenvironment. Cell Res, 2014. 24(12): p. 1420–32.
Zuppinger, C., M. Eppenberger-Eberhardt, and H.M. Eppenberger, N-Cadherin: structure, function and importance in the formation of new intercalated disc-like cell contacts in cardiomyocytes. Heart Fail Rev, 2000. 5(3): p. 251–7.
Wahl, J.K., 3rd, et al., N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem, 2003. 278(19): p. 17269–76.
Kim, J.B., et al., N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J Cell Biol, 2000. 151(6): p. 1193–206.
Mrozik, K.M., et al., N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer, 2018. 18(1): p. 939.
Corcoran, K.E., et al., Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS One, 2007. 3: p. e2563.
Singh, D., et al., Increased expression of preprotachykinin-I and neurokinin receptors in human breast cancer cells: implications for bone marrow metastasis. Proc Natl Acad Sci U S A, 2000. 97(1): p. 388–93.
Ghazaryan, S., et al., Inactivation of Rb and E2f8 synergizes to trigger stressed DNA replication during erythroid terminal differentiation. Mol Cell Biol, 2014. 34(15): p. 2833–47.
Qian, J., et al., Cloning of human preprotachykinin-I promoter and the role of cyclic adenosine 5′
-monophosphate response elements in its expression by IL-1 and stem cell factor. J Immunol, 2001. 166(4): p. 2553–61.
Kotini, M., et al., Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat Commun, 2018. 9(1): p. 3846.
Park, J.M., et al., Exogenous CXCL12 activates protein kinase C to phosphorylate connexin 43 for gap junctional intercellular communication among confluent breast cancer cells. Cancer Letters, 2013. 331(1): p. 84–91.
Ramamoorthi, G., et al., Disseminated cancer cells in breast cancer: Mechanism of dissemination and dormancy and emerging insights on therapeutic opportunities. Seminars in Cancer Biology, 2022. 78: p. 78–89.
Ho, Y.-H. and S. Méndez-Ferrer, Microenvironmental contributions to hematopoietic stem cell aging. Haematologica, 2020. 105(1): p. 38–46.
Buijs, J.T., et al., The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene, 2012. 31(17): p. 2164–74.
Yang, H., et al., TGF-beta-activated SMAD3/4 complex transcriptionally upregulates N-cadherin expression in non-small cell lung cancer. Lung Cancer, 2015. 87(3): p. 249–57.
Oh, H.S., et al., Bone marrow stroma influences transforming growth factor-beta production in breast cancer cells to regulate c-myc activation of the preprotachykinin-I gene in breast cancer cells. Cancer Res, 2004. 64(17): p. 6327–36.
Park, J.M., et al., Exogenous CXCL12 activates protein kinase C to phosphorylate connexin 43 for gap junctional intercellular communication among confluent breast cancer cells. Cancer Lett, 2013. 331(1): p. 84–91.
Singh, A.K. and J.A. Cancelas, Gap Junctions in the Bone Marrow Lympho-Hematopoietic Stem Cell Niche, Leukemia Progression, and Chemoresistance. Int J Mol Sci, 2020. 21(3).
Li, F., et al., Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell, 2011. 20(3): p. 291–302.
Niessen, C.M., D. Leckband, and A.S. Yap, Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol Rev, 2011. 91(2): p. 691–731.
Chen, C.R., et al., E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell, 2002. 110(1): p. 19–32.