Therapeutic Immunomodulation with Mesenchymal Stromal Cells: The Need for In Vivo Clues
DOI:
https://doi.org/10.13052/ijts2246-8765.2015.003Keywords:
Mesenchymal stem/stromal cell, immunomodulation, immunosuppression, tolerance, scaffold, regenerative medicineAbstract
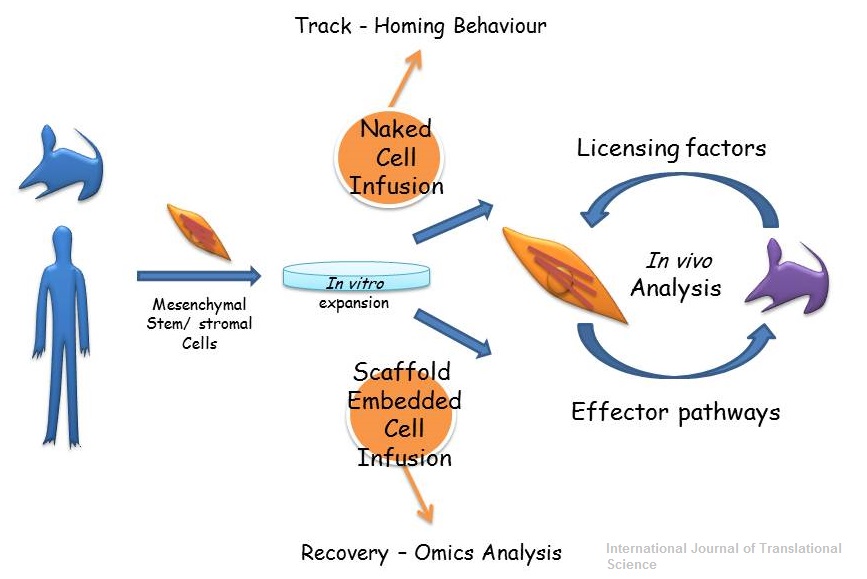
Mesenchymal stem/stromal cells are effective therapeutic agents for a variety of pathological conditions. However, the molecular mechanisms underlying their action remain largely unknown and biased by in vitro studies. In this concise review we have described recent advances in MSC therapeutics based on in vivo observations. We have also discussed the possibility of using engineering approaches to improve and facilitate deciphering MSC functions.
Downloads
References
A. J. Friedenstein, R. K. Chailakhjan, and K. S. Lalykina. “The development of fibroblast colonies in monolayer cultures of guineapig bone marrow and spleen cells.” Cell Tissue Kinet 3, 393–403. (1970).
E. Schipani and H. M. Kronenberg. “Adult mesenchymal stem cells,” in StemBook, ed Cambridge (MA): Harvard Stem Cell Institute Copyright: (c) 2008 Ernestina Schipani and Henry M. Kronenberg. (2008).
A. I. Caplan. “Adult mesenchymal stem cells for tissue engineering versus regenerative medicine.” J. Cell Physiol. 213, 341–347. (2007).
J. Kramer, F. Dazzi, M. Dominici, P. Schlenke, and W. Wagner. “Clinical perspectives of mesenchymal stem cells.” Stem Cells Int. 2012, 684827. (2012).
D. T. Shih and T. Burnouf. “Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion.” N. Biotechnol. 32, 199–211. (2015).
W. E. Fibbe, F. Dazzi, and K. LeBlanc. “MSCs: science and trials.” Nat. Med. 19, 812–813. (2013).
E. Jones and R. Sch¨afer. “Biological differences between native and cultured mesenchymal stem cells: implications for therapies.” Methods Mol. Biol. 1235, 105–120. (2015).
M. F. Pittenger, A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, et al. “Multilineage potential of adult human mesenchymal stem cells.” Science 284, 143–147. (1999).
F. J. Lv, R. S. Tuan, K. M. Cheung, and V. Y. Leung. “Concise review: the surface markers and identity of human mesenchymal stem cells.” Stem Cells 32, 1408–1419. (2014).
M. Maleki, F. Ghanbarvand, M. Reza Behvarz, M. Ejtemaei, and E. Ghadirkhomi. “Comparison of mesenchymal stem cell markers in multiple human adult stem cells.” Int. J. Stem Cells 7, 118–126. (2014).
R. Hass, C. Kasper, S. B¨ohm, and R. Jacobs. “Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC.” Cell Commun. Signal 9, 2. (2011).
A. Shaer, N. Azarpira, M. H. Aghdaie, and E. Esfandiari. “Isolation and characterization of Human Mesenchymal Stromal Cells Derived from Placental Decidua Basalis; Umbilical cord Wharton’s Jelly and Amniotic Membrane.” Pak. J. Med. Sci. 30, 1022–1026. (2014).
E. Collins, F. Gu, M. Qi, I. Molano, P. Ruiz, L. Sun, et al. “Differential efficacy of human mesenchymal stem cells based on source of origin,” J. Immunol. 193, 4381–4390. (2014).
W. Liu, F. Song, L. Ren, W. Guo, T. Wang, Y. Feng, et al. “The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases.” J. Cell Mol. Med. (2014).
M. J. Goumans, J. A. Maring, and A. M. Smits. “A straightforward guide to the basic science behind cardiovascular cell-based therapies.” Heart 100, 1153–1157. (2014).
J. Bashir, A. Sherman, H. Lee, L. Kaplan, and J. M. Hare. “Mesenchymal stem cell therapies in the treatment of musculoskeletal diseases.” PMR 6, 61–69. (2014).
M. Baghaban Eslaminejad and E. Malakooty Poor. “Mesenchymal stem cells as a potent cell source for articular cartilage regeneration,” World J. Stem Cells 6, 344–354. (2014).
B. Kristj´ansson and S. Honsawek. “Current perspectives in mesenchymal stem cell therapies for osteoarthritis.” Stem Cells Int. 2014, 194318. (2014).
J. F. Swart and N. M. Wulffraat. “Mesenchymal stromal cells for treatment of arthritis.” Best Pract. Res. Clin. Rheumatol. 28, 589–603. (2014).
Y. Liu, J. Wu, Y. Zhu, and J. Han. “Therapeutic application of mesenchy-mal stem cells in bone and joint diseases,” Clin. Exp. Med. 4, 13–24. (2014).
B. Skovrlj, G. Cunn, J. Guzman, and S. A. Qureshi. “Mesenchymal stem cell technology in the treatment of degenerative disc disease.” J. Neurosurg. Sci. (2014).
M. Boido, A. Piras, V. Valsecchi, G. Spigolon, K. Mareschi, I. Ferrero, et al. “Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis.” Cytotherapy 16, 1059–1072. (2014).
L. Mazzini, K. Mareschi, I. Ferrero, M. Miglioretti, A. Stecco, S. Servo, et al. “Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study.” Cytotherapy 14, 56–60. (2012).
K. A. Chang, H. J. Kim, Y. Joo, S. Ha, and Y. H. Suh. “The therapeutic effects of human adipose-derived stem cells in Alzheimer’s disease mouse models.” Neurodegener. Dis. 13, 99–102. (2014).
N. Kim and S. G. Cho. “Clinical applications of mesenchymal stem cells.” Korean J. Int. Med. 28, 387–402. (2013).
E. Buzhor, L. Leshansky, J. Blumenthal, H. Barash, D. Warshawsky, Y. Mazor, et al. “Cell-based therapy approaches: the hope for incurable diseases.” Regen Med. 9, 649–672. (2014).
K. Le Blanc, F. Frassoni, L. Ball, F. Locatelli, H. Roelofs, I. Lewis, et al. “Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study.” Lancet 371, 1579–1586. (2008).
J. Tan, W. Wu, X. Xu, L. Liao, F. Zheng, S. Messinger, et al. “Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial.” JAMA 307, 1169–77. (2012).
R. Ciccocioppo, M. E. Bernardo, A. Sgarella, R. Maccario, M. A. Avanzini, C. Ubezio, et al. “Autologous bone marrow-derived mesenchy- mal stromal cells in the treatment of fistulising Crohn’s disease.” Gut 60, 788–798. (2011).
P. Connick, M. Kolappan, C. Crawley, D. J. Webber, R. Patani, A. W. Michell, et al. “Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study.” Lancet Neurol. 11, 150–156. (2012).
I. M¨uller, S. Lymperi, and F. Dazzi. “Mesenchymal stem cell therapy for degenerative inflammatory disorders.” Curr. Opin. Organ. Transplant. 13, 639–644. (2008).
D. Polchert, J. Sobinsky, G. Douglas, M. Kidd, A. Moadsiri, E. Reina, et al. “IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease.” Eur. J. Immunol. 38,
–1755. (2008).
G. Ren, L. Zhang, X. Zhao, G. Xu, Y. Zhang, A. I. Roberts, et al. “Mes-enchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide.” Cell Stem Cell 2, 141–150. (2008).
M. Krampera. “Mesenchymal stromal cell ‘licensing’: a multistep process.” Leukemia 25, 1408–414. (2011).
L. Wang, Y. Zhao, and S. Shi. “Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration.” J. Dent. Res. 91, 1003–1010. (2012).
I. Marigo and F. Dazzi. “The immunomodulatory properties of mes-enchymal stem cells.” Semin. Immunopathol. 33, 593–602. (2011).
P. Renner, E. Eggenhofer, A. Rosenauer, F. C. Popp, J. F. Steinmann, P. Slowik, et al. “Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function.” Transplant Proc. 41, 2607–2611. (2009).
E. Valencic, C. Loganes, S. Cesana, E. Piscianz, G. Gaipa, E. Biagi, et al. “Inhibition of mesenchymal stromal cells by pre-activated lymphocytes and their culture media.” Stem Cell Res. Ther. 5, 3. (2014).
J. Su, X. Chen, Y. Huang, W. Li, J. Li, K. Cao, et al. “Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species.” Cell Death Differ. 21, 388–396. (2014).
G. Ren, J. Su, L. Zhang, X. Zhao, W. Ling, A. L’huillie, et al. “Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression.” Stem Cells 27, 1954–1962. (2009).
R. Meisel, S. Brockers, K. Heseler, O. Degistirici, H. B¨ulle, C. Woite, et al. “Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase.” Leukemia 25, 648–654. (2011).
K. Schroder, K. M. Irvine, M. S. Taylor, N. J. Bokil, K. A. Le Cao, K. A. Masterman, et al. “Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages.” Proc. Natl. Acad Sci. U.S.A. 109, E944–E953. (2012).
M. Orciani, A. Campanati, E. Salvolini, G. Lucarini, G. Di Benedetto, A. Offidani, et al. “The mesenchymal stem cell profile in psoriasis.” Br. J. Dermatol. 165, 585–592. (2011).
H. J. Ball, F. F. Jusof, S. M. Bakmiwewa, N. H. Hunt, and H. J. Yuasa. “Tryptophan-catabolizing enzymes - party of three.” Front. Immunol. 5:485. (2014).
K. English, F. P. Barry, C. P. Field-Corbett, and B. P. Mahon. “IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells.” Immunol. Lett. 110, 91–100. (2007).
H. Y. Jui, C. H. Lin, W. T. Hsu, Y. R. Liu, R. B. Hsu, B. L. Chiang, et al. “Autologous mesenchymal stem cells prevent transplant arterio-sclerosis by enhancing local expression of interleukin-10, interferon-γ, and indoleamine 2,3-dioxygenase.” Cell Transplant 21, 971–984. (2012).
W. Ge, J. Jiang, J. Arp, W. Liu, B. Garcia, and H. Wang. “Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression.” Transplantation 90, 1312–1320. (2010).
D. Wang, X. Feng, L. Lu, J. E. Konkel, H. Zhang, Z. Chen, et al. “A CD8 T cell/indoleamine 2,3-dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus.” Arthritis Rheumatol. 66, 2234–2245. (2014).
D. Chabannes, M. Hill, E. Merieau, J. Rossignol, R. Brion, J. P. Soulillou, et al. “A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells.” Blood 110, 3691–3694. (2007).
C. Bouffi, C. Bony, G. Courties, C. Jorgensen, and D. No¨el. “IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis,” PLoS ONE 5:e14247. (2010).
L. Zhang, R. J. Dang, H. Li, P. Li, Y. M. Yang, X. M. Guo, et al. “SOCS1 regulates the immune modulatory properties of mesenchymal stem cells by inhibiting nitric oxide production.” PLoS ONE 9:e97256. (2014).
Y. Huang, P. Yu, W. Li, G. Ren, A. I. Roberts, W. Cao, et al. “p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation.” Oncogene 33, 3830–3838. (2014).
D. Claar, T. V. Hartert, and R. S. Peebles. “The role of prostaglandins in allergic lung inflammation and asthma.” Expert Rev. Respir Med. 1–18. (2014).
K. Kawahara, H. Hohjoh, T. Inazumi, S. Tsuchiya, and Y. Sugimoto. “Prostaglandin E2-induced inflammation: Relevance of prostaglandin Ereceptors.” Biochim. Biophys. Acta (2014).
P. Kalinski. “Regulation of immune responses by prostaglandin E2.” J. Immunol. 188, 21–28. (2012).
Y. C. Wang, S. H. Wang, Y. N. Wei, D. W. Du, H. Xu, C. C. Gao, et al. “Notch-RBP-J signaling is required by bone marrow stromal cells for the treatment of acute graft versus host disease.” Stem Cell Res. 11, 721–735. (2013).
T. J. Bartosh, J. H. Yl¨ostalo, N. Bazhanov, J. Kuhlman, and D. J. Prockop. “Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1).” Stem Cells 31, 2443–2456. (2013).
Y. Qiu, M. M. Yun, X. Han, R. Zhao, E. Zhou, and S. Yun. “Human umbilical cord mesenchymal stromal cells suppress MHC class II expression on rat vascular endothelium and prolong survival time of cardiac allograft.” Int. J. Clin. Exp. Med. 7, 1760–1767. (2014).
M. Koch, A. Lehnhardt, X. Hu, B. Brunswig-Spickenheier, M. Stolk, V. Br¨ocker, et al. “Isogeneic MSC application in a rat model of acute renal allograft rejection modulates immune response but does not prolong allograft survival.” Transpl. Immunol. 29, 43–50. (2013).
R. A. Larocca, P. M. Moraes-Vieira, E. J. Bassi, P. Semedo, D. C. de Almeida, M. B. da Silva, et al. “Adipose tissue-derived mesenchymal stem cells increase skin allograft survival and inhibit Th-17 immune response.” PLoS ONE 8:e76396. (2013).
M. A. Gonz´alez, E. Gonzalez-Rey, L. Rico, D. B¨uscher, and M. Delgado. “Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses.” Gastroenterology 136, 978–989. (2009).
E. Gonzalez-Rey, P. Anderson, M. A. Gonz´alez, L. Rico, D. B¨uscher, and M. Delgado. “Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis.” Gut 58, 929–939. (2009).
Q. Q. Chen, L. Yan, C. Z. Wang, W. H. Wang, H. Shi, B. B. Su, et al. “Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses.” World J. Gastroenterol. 19, 4702–4717. (2013).
H. Li, Z. Guo, H. Zhu, X. S. Li, X. Jiang, H. Yao, et al. “Transplanted mesenchymal stem cells can inhibit the three developmental stages of murine acute graft-versus-host disease.” In Vivo 24, 659–666. (2010).
S. Chiesa, S. Morbelli, S. Morando, M. Massollo, C. Marini, A. Bertoni, et al. “Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells.” Proc. Natl. Acad Sci. U.S.A. 108, 17384–1739. (2011).
H. Li, Z. Guo, X. Jiang, H. Zhu, X. Li, and N. Mao. “Mesenchymal stem cells alter migratory property of T and dendritic cells to delay thedevelopment of murine lethal acute graft-versus-host disease.” Stem Cells 26, 2531–2541. (2008).
M. G. Kim, S. H. Kim, H. Noh, Y. S. Ko, H. Y. Lee, S. K. Jo, et al. “CD11c+ cells partially mediate the renoprotective effect induced by bone marrow-derived mesenchymal stem cells.” PLoS ONE 8:e72544. (2013).
Y. Geng, L. Zhang, B. Fu, J. Zhang, Q. Hong, J. Hu, et al. “Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages.” Stem Cell Res. Ther. 5, 80. (2014).
D. I. Cho, M. R. Kim, H. Y. Jeong, H. C. Jeong, M. H. Jeong, S. H. Yoon, et al. “Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages.” Exp. Mol. Med. 46:e70. (2014).
B. L. Yen, M. L. Yen, P. J. Hsu, K. J. Liu, C. J. Wang, C. H. Bai, et al. “Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3.” Stem Cell Rep. 1, 139–151. (2013).
H. W. Chen, H. Y. Chen, L. T. Wang, F. H. Wang, L. W. Fang, H. Y. Lai, et al. “Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines.” J. Immunol. 190, 5065–5077. (2013).
X. Su, L. Zhang, J. Ye, L. Yang, Y. Li, and Y. Wang. “Bone marrow mesenchymal stem cells suppress ascitogenous hepatoma progression in BALB/c mouse through reducing myeloid-derived suppressor cells.” Biomed. Mater Eng. 25, 167–177. (2015).
L. Senseb´e and S. Fleury-Cappellesso. “Biodistribution of mesenchymal stem/stromal cells in a preclinical setting.” Stem Cells Int. 2013, 678063. (2013).
H. S. Kim, J. Woo, Y. Choi, E. H. Hwang, S. K. Choi, K. W. Cho, et al. “Noninvasive MRI and multilineage differentiation capability of ferritin-transduced human mesenchymal stem cells.” NMR Biomed (2014).
K. Geng, Z. X. Yang, D. Huang, M. Yi, Y. Jia, G. Yan, et al. “Tracking of mesenchymal stem cells labeled with gadolinium diethylenetriamine pentaacetic acid by 7T magnetic resonance imaging in a model of
cerebral ischemia.” Mol. Med. Rep. 11, 954–960. (2015).
P. Hua, Y. Y. Wang, L. B. Liu, J. L. Liu, J. Y. Liu, Y. Q. Yang, et al. “In vivo magnetic resonance imaging tracking of transplanted superparamagnetic iron oxidelabeled bone marrow mesenchymal stem cells in rats with myocardial infarction.” Mol. Med. Rep. 11, 113–1120. (2015).
Jasmin, L. A. Jelicks, H. B. Tanowitz, V. M. Peters, R. Mendez-Otero, A. C. Campos de Carvalho, et al. “Molecular imaging, biodistribution and efficacy of mesenchymal bone marrow cell therapy in a mouse model of Chagas disease.” Microbes Infect 16, 923–935. (2014).
A. Gholamrezanezhad, S. Mirpour, M. Bagheri, M. Mohamadnejad, K. Alimoghaddam, L. Abdolahzadeh, et al. “In vivo tracking of 111In- oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis.” Nucl. Med. Biol. 38, 961–917. (2011).
O. Betzer, A. Shwartz, M. Motiei, G. Kazimirsky, I. Gispan, E. Damti, et al. “Nanoparticle-based CT imaging technique for longitudinal and quantitative stem cell tracking within the brain: application in neuropsy-chiatric disorders.” ACS Nano 8, 9274–9285. (2014).
E. Wolfs, T. Struys, T. Notelaers, S. J. Roberts, A. Sohni, G. Bormans, et al. ”18F-FDG labeling of mesenchymal stem cells and multipotent adult progenitor cells for PET imaging: effects on ultrastructure and differentiation capacity.” J. Nucl. Med. 54, 447–454. (2013).
M. Kantarcioglu, B. Caliskan, H. Demirci, O. Karacalioglu, M. Kekilli, Z. Polat, et al. “The efficacy of mesenchymal stem cell transplantation in caustic esophagus injury: an experimental study.” Stem Cells Int. 2014, 939674. (2014).
M. Hofmann, K. C. Wollert, G. P. Meyer, A. Menke, L. Arseniev, B. Hertenstein, et al. “Monitoring of bone marrow cell homing into the infarcted human myocardium.” Circulation 111, 2198–2202. (2005).
T. Garg, O. Singh, S. Arora, and R. Murthy. “Scaffold: a novel carrier for cell and drug delivery.” Crit. Rev. Ther. Drug Carrier Syst. 29, 1–63. (2012).
A. Goren, N. Dahan, E. Goren, L. Baruch, and M. Machluf. “Encapsulated human mesenchymal stem cells: a unique hypoimmunogenic platform for long-term cellular therapy.” FASEB J. 24, 22–31. (2010).
J. F. Markusen, C. Mason, D. A. Hull, M. A. Town, A. B. Tabor, M. Clements, et al. “Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads.” Tissue Eng. 12, 821–830. (2006).
J. Barminko, J. H. Kim, S. Otsuka, A. Gray, R. Schloss, M. Grumet, et al. “Encapsulated mesenchymal stromal cells for in vivo transplantation.” Biotechnol. Bioeng. 108, 2747–2758. (2011).
L. Zanotti,A. Sarukhan, E. Dander, M. Castor, J. Cibella, C. Soldani, et al. “Encapsulated mesenchymal stem cells for in vivo immunomodulation.” Leukemia 27, 500–503. (2013).
R. P. Meier, R. Mahou, P. Morel, J. Meyer, E. Montanari, Y. D. Muller, et al. “Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice.” J. Hepatol. (2014).
X. Zhang, K. Yamaoka, K. Sonomoto, H. Kaneko, M. Satake, Y. Yamamoto, et al. “Local delivery of mesenchymal stem cells with poly-lactic-co-glycolic Acid nano-fiber scaffold suppress arthritis in rats.” PLoS ONE 9:e114621. (2014).
S. J. Hwang, T. H. Cho, and I. S. Kim. “In vivo gene activity of human mesenchymal stem cells after scaffold-mediated local transplantation.” Tissue Eng Part A 20, 2350–2364. (2014).
C. H. Chen, H. J. Wei, W. W. Lin, I. Chiu, S. M. Hwang, C. C. Wang, et al. “Porous tissue grafts sandwiched with multilayered mesenchymal stro- mal cell sheets induce tissue regeneration for cardiac repair.” Cardiovasc Res. 80, 88–95. (2008).
J. H. Brauker, V. E. Carr-Brendel, L. A. Martinson, J. Crudele, W. D. Johnston, and R. C. Johnson. “Neovascularization of synthetic mem- branes directed by membrane microarchitecture.” J. Biomed. Mater Res. 29, 1517–1524. (1995).
T. A. Telemeco, C. Ayres, G. L. Bowlin, G. E. Wnek, E. D. Boland, N. Cohen, et al. “Regulation of cellular infiltration into tissue engi- neering scaffolds composed of submicron diameter fibrils produced by electrospinning.” Acta Biomater. 1, 377–385. (2005).
B. J. Lawrence and S. V. Madihally. “Cell colonization in degradable 3D porous matrices.” Cell Adh. Migr. 2, 9–16. (2008).