Developing Therapies with Functional Beta Cells to Treat Diabetes
DOI:
https://doi.org/10.13052/ijts2246-8765.20151004Abstract
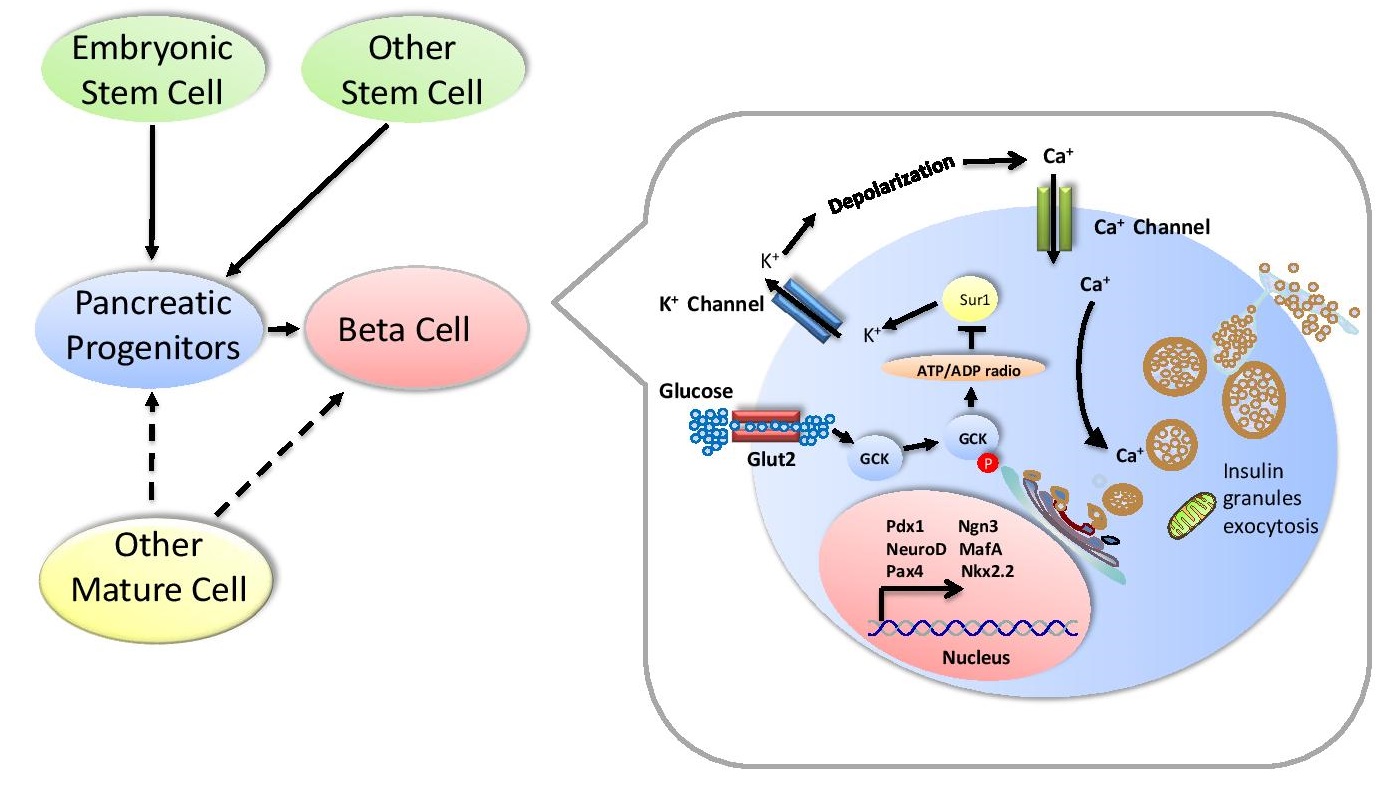
As the key regulator in maintaining blood sugar, insulin is secreted by beta cells residing in pancreatic islets. However, most functional beta cells are disap- peared in type I diabetes, and the total beta cell population is seriously reduced in type II diabetes. Over the last decade, insights into beta cell development, combined with the deeper research of related donor stem cells including ESCs, iPSCs and pancreatic progenitors, have led us to generate functional beta cells. Besides, several alternative approaches have also converted other cell types into insulin positive beta-like cells via lineage reprogramming factors (Pdx1, Nkx6-1, Pax4 and Ngn3). Nowadays, people realized that advances in investigating of beta cell biology at the genomic and post-transcription levels are more useful in deeply understanding the cells source, behavior and function. For research in drug screening or diabetes transplantation therapy, herein, we reviewed the recent information about development, replication of beta cells, and production of insulin-positive cells via lineage reprogramming or small molecule treatment.
Downloads
References
Petkov, P. E., and A. Vitanov. 1962. Development of connective tissue near the pancreatic islets of Langerhans in the human fetus. Nauchni
Tr. Vissh. Med. Inst. Sofiia 41: 91–108.
Heinze, E., and J. Steinke. 1972. Insulin secretion during development: response of isolated pancreatic islets of fetal, newborn and adult rats to theophylline and arginine. Horm. Metab. Res. 4(4): 234–236. 54 Y. Zhao
Naya, F. J., H. P. Huang, Y. Qiu, H. Mutoh, F. J. DeMayo, A. B. Leiter, and M. J. Tsai. 1997. Diabetes, defective pancreatic morphogenesis, and
abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 11(18): 2323–2334.
Yamaoka, T., and M. Itakura. 1999. Development of pancreatic islets (review). Int. J. Mol. Med. 3(3): 247–261.
Nathan, D. M. 1993. Long-term complications of diabetes mellitus. N. Engl. J. Med. 328(23): 1676–1685.
Daneman, D. 2002. Islet cell transplantation and other new technologies for treating type 1 diabetes: a paediatric view. Horm. Res. 57(1): 54–59.
Serup, P., O. D. Madsen, and T. Mandrup-Poulsen. 2001. Islet and stem cell transplantation for treating diabetes. BMJ 322: 29–32.
Liu, E. H., K. I. Rother, and D. M. Harlan. 2005. Islet transplantation and the challenges of treating type 1 diabetes. Discov. Med. 5(25): 43–49.
Yang, L., S. Li, H. Hatch, K. Ahrens, J. G. Cornelius, B. E. Petersen, and A. B. Peck.2002. In vitro trans-differentiation of adult hepatic stem cells
into pancreatic endocrine hormone-producing cells. Proc. Natl. Acad. Sci. USA, 99(12): 8078–8083.
Lacy, P. E. and D. W. Scharp. 1986. Islet transplantation in treating diabetes. Annu. Rev. Med. 37: 33–40.
Efrat, S. 2008. Beta-cell replacement for insulin-dependent diabetes mellitus. Adv. Drug. Deliv. Rev. 60(2): 114–123.
Collombat, P., X. Xu, H. Heimberg, and A. Mansouri. 2010. Pancreatic beta-cells: from generation to regeneration. Semin. Cell Dev. Biol. 21(8); 838–844.
Ashcroft, F. M., and P. Rorsman. 2012. Diabetes mellitus and the beta cell: the last ten years. Cell, 148(6): 1160–1171.
Ben-Othman, N., M. Courtney, A. Vieira, A. Pfeifer, N. Druelle, E. Gjernes, B. Faurite, F. Avolio, and P. Collombat. 2013. From pancre-
atic islet formation to beta-cell regeneration. Diabetes Res. Clin. Pract. 101(1): 1–9.
Borowiak, M., R. Maehr, S. Chen, A. E. Chen, W. Tang, J. L. Fox, S. L. Schreiber, and D. A. Melton. 2009. Small molecules efficiently
direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell, 4(4): 348–358.
Oliver-Krasinski, J. M., and D. A. Stoffers. On the origin of the beta cell. Genes Dev. 22(15): 1998–2021.
Desgraz, R., C. Bonal, and P. L. Herrera. 2011. beta-cell regeneration: the pancreatic intrinsic faculty. Trends Endocrinol. Metab. 22(1): 34–43. Developing Therapies with Functional Beta Cells to Treat Diabetes 55
Caro, J. J., A. J. Ward, and J. A. O’Brien. 2002. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care,
(3); 476–481.
Rhodes, C. J. 2005. Type 2 diabetes-a matter of beta-cell life and death?. Science, 307(5708): 380–384.
Kassem, S., S. Bhandari, P. Rodriguez-Bada, R. Motaghedi, M. Heyman, M. A. Garcia-Gimeno, N. Cobo-Vuilleumier, P. Sanz, N. K. Maclaren,
J. Rahier, B. Glaser, and A. L. Cuesta-Munoz. 2010. Large islets, beta-cell proliferation, and a glucokinase mutation. N. Engl. J. Med. 362(14):
–1350.
Cnop, M., N. Welsh, J. C. Jonas, A. Jorns, S. Lenzen, and D. L. Eizirik. 2005. Mechanisms of pancreatic beta-cell death in type 1 and type 2
diabetes: many differences, few similarities. Diabetes, 54(2): S97–S107.
Zhang, C., W. Bao, Y. Rong, H. Yang, K. Bowers, E. Yeung, and M. Kiely. 2016. Genetic variants and the risk of gestational diabetes mellitus: a systematic review. Hum. Reprod. Update, 19(4): 376–390.
Bluestone, J. A., K. Herold, and G. Eisenbarth. 2010. Genetics, patho- genesis and clinical interventions in type 1 diabetes. Nature, 464(7293): 1293–1300.
Liu, Y., A. Kumar, D. W. Boykin, and W. D. Wilson. 2007. Sequence and length dependent thermodynamic differences in heterocyclic diamidine interactions at AT base pairs in the DNA minor groove. Biophys. Chem. 131: 1–14.
Adrian, T. E., S. R. Bloom, K. Hermansen, and J. Iversen. Pancreatic polypeptide, glucagon and insulin secretion from the isolated perfused
canine pancreas. Diabetologia, 14(6): 413–417.
Sak, M. F., I. A. Macchi, and S. B. Beaser. 1965. Postnatal Development of Beta Cells and Ila Secretion in the Pancreatic Islets of the Golden
Hamster. Anat. Rec. 152: 25–33.
Vantyghem, M. C., F. Defrance, D. Quintin, C. Leroy, V. Raverdi, G. Prevost, R. Caiazzo, J. Kerr-Conte, F. Glowacki, M. Hazzan,
C. Noel, F. Pattou, A. S. Diamenord, R. Bresson, M. F. Bourdelle-Hego, M. Cazaubiel, M. Cordonnier, D. Delefosse, F. Dorey, A. Fayard,
C. Fermon, P. Fontaine, C. Gillot, S. Haye, A. C. Le Guillou, W. Karrouz, C. Lemaire, M. Lepeut, R. Leroy, B. Mycinski, E. Parent, C. Siame, A. Sterkers, F. Torres, O. Verier-Mine, E. Verlet, R. Desailloud, A. Durrbach, M. Godin, J. D. Lalau, C. Lukas-Croisier, E. Thervet, O. Toupance, Y. Reznik, and P. F. Westeel. 2014. Treating diabetes with 56 Y. Zhao islet transplantation: Lessons from the past decade in Lille. Diabetes
Metab. 40(2): 108–119.
Kornete, M., H. Beauchemin, C. Polychronakos, and C. A. Piccirillo. 2013. Pancreatic islet cell phenotype and endocrine function throughout diabetes development in non-obese diabetic mice. Autoimmunity, 46(4): 259–268.
Bocian-Sobkowska, J., M. Zabel, W. Wozniak, and J. Surdyk-Zasada. 1997. Prenatal development of the human pancreatic islets. Immuno-
cytochemical identification of insulin-, glucagon-, somatostatin- and pancreatic polypeptide-containing cells. Folia Histochem. Cytobiol.
(3): 151–154.
Kim, S. H., F. Abbasi, C. Lamendola, G. M. Reaven, and T. McLaughlin. 2010. Glucose-stimulated insulin secretion in gastric bypass patients
with hypoglycemic syndrome: no evidence for inappropriate pancreatic beta-cell function. Obes. Surg. 20(8); 1110–1116.
Spooner, B. S., B. T. Walther, and W. J. Rutter. 1970. The development of the dorsal and ventral mammalian pancreas in vivo and in vitro. J. Cell Biol. 47(1): 235–246.
Slack, J. M. 1995. Developmental biology of the pancreas. Development, 121(6): 1569–1580.
Kim, S. K. and D. A. Melton. 1998. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor. Proc. Natl. Acad. Sci. USA, 95(22): 13036–13041.
Pictet, R. L., W. R. Clark, R. H. Williams, and W. J. Rutter. An ultrastructural analysis of the developing embryonic pancreas. Dev. Biol.
(4): 436–467.
Edlund, H. 2001. Developmental biology of the pancreas. Diabetes, 50(1): 5–9.
Rieck, S., E. D. Bankaitis, and C. V. Wright. 2012. Lineage determinants in early endocrine development. Semin. Cell Dev. Biol. 23(6): 673–684.
Gu, G., J. Dubauskaite, and D. A. Melton. 2002. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct
from duct progenitors. Development, 129(10): 2447–2457.
Vignjevic, S., V. Todorovic, S. Damjanovic, M. Budec, O. Mitrovic, D. Djikic, N. Drndarevic, M. Micic, J. Miskovic-Krivokapic, S. Djuricic,
and I. Nikolic. 2012. Similar developmental patterns of ghrelin- and glucagon-expressing cells in the human pancreas. Cells Tissues Organs,
(4): 362–373. Developing Therapies with Functional Beta Cells to Treat Diabetes 57
Benitez, C. M., W. R. Goodyer, and S. K. Kim. 2012. Deconstructing pancreas developmental biology. Cold Spring Harb. Perspect. Biol. 4(6).
doi: 10.1101/cshperspect.a012401.
Chen, C. Z., L. Li, H. F. Lodish, and D. P. Bartel. 2004. MicroRNAs modulate hematopoietic lineage differentiation. Science, 303(5654):
–86.
Cano, D. A., B. Soria, F. Martin, and A. Rojas. 2014. Transcriptional control of mammalian pancreas organogenesis. Cell Mol. Life Sci.
(13): 2383–2402.
Offield, M. F., T. L. Jetton, P. A. Labosky, M. Ray, R. W. Stein, M.A. Magnuson, B. L. Hogan, and C. V. Wright. 1996. PDX-1 is required
for pancreatic outgrowth and differentiation of the rostral duodenum. 1996. Development, 122(3): 983–995.
McKinnon, C. M. and K. Docherty. 2001. Pancreatic duodenal homeobox-1, PDX-1, a major regulator of beta cell identity and function.
Diabetologia, 44(10): 1203–1214.
Holland, A. M., L. J. Gonez, G. Naselli, R. J. Macdonald, and L. C. Harrison. 2005. Conditional expression demonstrates the role
of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes, 54(9):
–2595.
Gannon, M., E. T. Ables, L. Crawford, D. Lowe, M. F. Offield, M. A. Magnuson, and C. V. Wright. 2008. pdx-1 function is specifically
required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev. Biol.
(2): 406–417.
Gao, N., J. LeLay, M. Z. Vatamaniuk, S. Rieck, J. R. Friedman, and K. H. Kaestner. 2008. Dynamic regulation of Pdx1 enhancers by Foxa1
and Foxa2 is essential for pancreas development. Genes Dev. 22(24): 3435–3448.
Kajiyama, H., T. S. Hamazaki, M. Tokuhara, S. Masui, K. Okabayashi, K. Ohnuma, S. Yabe, K. Yasuda, S. Ishiura, H. Okochi, and M. Asashima.
Pdx1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic
mice. Int. J. Dev. Biol. 54(4): 699–705.
Kaneto, H., Y. Nakatani, T. Miyatsuka, T. A. Matsuoka, M. Matsuhisa, M. Hori, and Y. Yamasaki. 2005. PDX-1/VP16 fusion protein, together
with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes, 54(4): 1009–1022.
Y. Zhao
Gradwohl, G., A. Dierich, M. LeMeur, and F. Guillemot. 2000. Neuro- genin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA, 97(4): 1607–1611.
Collombat, P., A. Mansouri, J. Hecksher-Sorensen, P. Serup, J. Krull, G. Gradwohl, and P. Gruss. 2003. Opposing actions of Arx and Pax4 in
endocrine pancreas development. Genes Dev. 17(20): 2591–2603.
Sosa-Pineda, B., K. Chowdhury, M. Torres, G. Oliver, and P. Gruss. 1997. The Pax4 gene is essential for differentiation of insulin-producing
beta cells in the mammalian pancreas. Nature, 386(6623): 399–402.
Itkin-Ansari, P., E. Marcora, I. Geron, B. Tyrberg, C. Demeterco, E. Hao, C. Padilla, C. Ratineau, A. Leiter, J. E. Lee, and F. Levine. 2005.
NeuroD1 in the endocrine pancreas: localization and dual function as an activator and repressor. Dev. Dyn. 233(3): 946–953.
Sussel, L., J. Kalamaras, D. J. Hartigan-O’Connor, J. J. Meneses, R. A. Pedersen, J. L. Rubenstein, and M. S. German. 1998. Mice lacking
the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development, 125(12):
–2221.
Sander, M., L. Sussel, J. Conners, D. Scheel, J. Kalamaras, F. Dela Cruz, V. Schwitzgebel, A. Hayes-Jordan, and M. German. 2000. Homeobox
gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development, 127(24): 5533–5540.
Henseleit, K. D., S. B. Nelson, K. Kuhlbrodt, J. C. Hennings, J. Ericson, and M. Sander. NKX6 transcription factor activity is required for alpha-
and beta-cell development in the pancreas. Development, 132(13): 3139–49.
Collombat, P., J. Hecksher-Sorensen, V. Broccoli, J. Krull, I. Ponte, T. Mundiger, J. Smith, P. Gruss, P. Serup, and A. Mansouri. 2005.
The simultaneous loss of Arx and Pax4 genes promotes a somatostatin- producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development, 132(13): 2969–2980.
St-Onge, L., B. Sosa-Pineda, K. Chowdhury, A. Mansouri, and P. Gruss. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature, 387(6631): 406–409.
Dohrmann, C., P. Gruss, and L. Lemaire. 2000. Pax genes and the differentiation of hormone-producing endocrine cells in the pancreas.
Mech. Dev. 92(1): 47–54. Developing Therapies with Functional Beta Cells to Treat Diabetes 59
Artner, I., Y. Hang, M. Mazur, T. Yamamoto, M. Guo, J. Lindner, M. A. Magnuson, and R. Stein. 2010. MafA and MafB regulate genes
critical to beta-cells in a unique temporal manner. Diabetes, 59(10): 2530–2539.
Nishimura, W., T. Kondo, T. Salameh, I. El Khattabi, R. Dodge, S. Bonner-Weir, and A. Sharma. 2006. A switch from MafB to MafA
expression accompanies differentiation to pancreatic beta-cells. Dev. Biol. 293(2): 526–539.
Zhang, C., T. Moriguchi, M. Kajihara, R. Esaki, A. Harada, H. Shimohata, H. Oishi, M. Hamada, N. Morito, K. Hasegawa, T. Kudo,
J. D. Engel, M. Yamamoto, and S. Takahashi. 2005. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell Biol. 25(12):
–4976.
Artner, I., J. Le Lay, Y. Hang, L. Elghazi, J. C. Schisler, E. Henderson, B. Sosa-Pineda, and R. Stein. MafB: an activator of the glucagon gene
expressed in developing islet alpha- and beta-cells. Diabetes, 55(2): 297–304.
Mastracci, T. L., K. R. Anderson, J. B. Papizan, and L. Sussel. 2013. Regulation of Neurod1 contributes to the lineage potential of Neuro-
genin3+ endocrine precursor cells in the pancreas. PLoS Genet. 9(2): e1003278.
Brolen, G. K., N. Heins, J. Edsbagge, and H. Semb. 2005. Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing beta-cell-like cells. Diabetes, 54(10): 2867–2874.
D’Amour, K. A., A. D. Agulnick, S. Eliazer, O. G. Kelly, E. Kroon, and E. E. Baetge. Efficient differentiation of human embryonic stem cells to
definitive endoderm. Nat. Biotechnol. 23(12): 1534–1541.
D’Amour, K. A., A. G. Bang, S. Eliazer, O. G. Kelly, A. D. Agulnick, N. G. Smart, M. A. Moorman, E. Kroon, M. K. Carpenter, and E. E.
Baetge. 2006. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24(11):
–1401.
Kroon, E., L. A. Martinson, K. Kadoya, A. G. Bang, O. G. Kelly, S. Eliazer, H. Young, M. Richardson, N. G. Smart, J. Cunningham,
A. D.Agulnick, K.A. D’Amour, M. K. Carpenter, and E. E. Baetge. 2008. Pancreatic endoderm derived from human embryonic stem cells gener-
ates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26(4): 443–452. 60 Y. Zhao
Zhang, D., W. Jiang, M. Liu, X. Sui, X. Yin, S. Chen, Y. Shi, and H. Deng. Highly efficient differentiation of human ES cells and iPS
cells into mature pancreatic insulin-producing cells. Cell Res. 19(4): 429–438.
Huang, P., Z. He, S. Ji, H. Sun, D. Xiang, C. Liu, Y. Hu, X. Wang, and L. Hui. 2011. Induction of functional hepatocyte-like cells from mouse
fibroblasts by defined factors. Nature, 475(7356): 386–389.
Sapir, T., K. Shternhall, I. Meivar-Levy, T. Blumenfeld, H. Cohen, E. Skutelsky, S. Eventov-Friedman, I. Barshack, I. Goldberg, S. Pri-Chen, L. Ben-Dor, S. Polak-Charcon, A. Karasik, I. Shimon, E. Mor, and S. Ferber. 2005. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc. Natl. Acad. Sci. USA, 102(22): 7964–7969.
Zaret, K. S., and M. Grompe. 2008. Generation and regeneration of cells of the liver and pancreas. Science, 322(5907): 1490–1494.
Chung, C. H., E. Hao, R. Piran, E. Keinan, and F. Levine 2010. Pancreatic beta-cell neogenesis by direct conversion from mature alpha-cells. Stem Cells, 28(9): 1630–1638.
Thorel, F., and P. L. Herrera. 2010. Conversion of adult pancreatic alpha-cells to beta-cells in diabetic mice. Med. Sci. (Paris) 26(11): 906–909.
Collombat, P., X. Xu, P. Ravassard, B. Sosa-Pineda, S. Dussaud, N. Billestrup, O. D. Madsen, P. Serup, H. Heimberg, and A. Mansouri.
The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell, 138(3):
–462.
Ball, G. D., M. J. Weigensberg, M. L. Cruz, G. Q. Shaibi, H. A. Kobaissi, and M. I. Goran. 2005. Insulin sensitivity, insulin secretion and beta-cell function during puberty in overweight Hispanic children with a family history of type 2 diabetes. Int. J. Obes. (Lond) 29(12): 1471–1477.
Newsholme, P., V. Cruzat, F. Arfuso, and K. Keane. 2014. Nutrient regulation of insulin secretion and action. J. Endocrinol. 221(3):
R105–R120.
Rhodes, C. J., and M. F. White. 2002. Molecular insights into insulin action and secretion. Eur. J. Clin. Invest. 32(3): 3–13.
Wiederkehr, A., and C. B. Wollheim. 2006. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology, 147(6):
–9. Developing Therapies with Functional Beta Cells to Treat Diabetes 61
Jacobson, D. A., and L. H. Philipson. 2007. Action potentials and insulin secretion: new insights into the role of Kv channels. Diabetes. Obes. Metab. 9(2): 89–98.
Miyazaki, Y., H. Akasaka, H. Ohnishi, S. Saitoh, R. A. DeFronzo, and K. Shimamoto. 2008. Differences in insulin action and secretion, plasma
lipids and blood pressure levels between impaired fasting glucose and impaired glucose tolerance in Japanese subjects. Hypertens. Res. 31(7):
–1363.
Koster, J. C., M. A. Permutt, and C. G. Nichols. 2005. Diabetes and insulin secretion: the ATP-sensitive K+ channel (K ATP) connection.
Diabetes, 54(11): 3065–3072.
Martin-Gronert, M. S. and S. E. Ozanne. 2012. Metabolic programming of insulin action and secretion. Diabetes Obes. Metab, 14(3): 29–39.
Ambros, V. 2004. The functions of animal microRNAs. Nature, 431(7006): 350–355.
Joglekar, M. V., V. S. Parekh, and A. A. Hardikar. 2011. Islet-specific microRNAs in pancreas development, regeneration and diabetes. Indian J. Exp. Biol, 49(6): 401–408.
Joglekar, M. V., V. M. Joglekar, and A. A. Hardikar. 2009. Expression of islet-specific microRNAs during human pancreatic development. Gene. Expr. Patterns, 9(2): 109–113.
Xia, H. Q., Y. Pan, J. Peng, and G. X. Lu. 2011. Over-expression of miR375 reduces glucose-induced insulin secretion in Nit-1 cells. Mol.
Biol. Rep. 38(5): 3061–3065.
Poy, M. N., L. Eliasson, J. Krutzfeldt, S. Kuwajima, X. Ma, P. E. Macdonald, S. Pfeffer, T. Tuschl, N. Rajewsky, P. Rorsman, and
M. Stoffel. 2004. A pancreatic islet-specific microRNA regulates insulin secretion. Nature, 432(7014): 226–230.
Pullen, T. J., G. da Silva Xavier, G. Kelsey, and G. A. Rutter. 2011. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing
of monocarboxylate transporter 1 (Mct1). Mol. Cell. Biol. 31(15): 3182–3194.
Baroukh, N., M. A. Ravier, M. K. Loder, E. V. Hill, A. Bounacer, R. Scharfmann, G. A. Rutter, and E. Van Obberghen. 2007. MicroRNA-
a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J. Biol. Chem. 282(27): 19575–19588.
Roggli, E., A. Britan, S. Gattesco, N. Lin-Marq, A. Abderrahmani, P. Meda, and R. Regazzi. 2010. Involvement of microRNAs in the