Long Term Rejuvenation of Facial Skin by Transplantation of Autologous Stromal Vascular Fraction and Pre-conditioned Matrix: A Clinical Study
DOI:
https://doi.org/10.13052/ijts2246-8765.2024.021Keywords:
Clinical study, SVF, stem cells, hypoxiaAbstract
Background: For skin rejuvenation, today no procedure describes a specific treatment of the extracellular matrix (ECM) combined with stromal vascular fraction (SVF) from adipose tissue. A new product AmeaCell® , allows SVF extraction and ECM preconditioning in hypoxic conditions. Hypoxia significantly increased the adhesion of adipose derived stem cells and enhace their proliferation and differentiation.
Objective: This clinical study aimed to evaluate safety and efficiency of this new procedure, over one year.
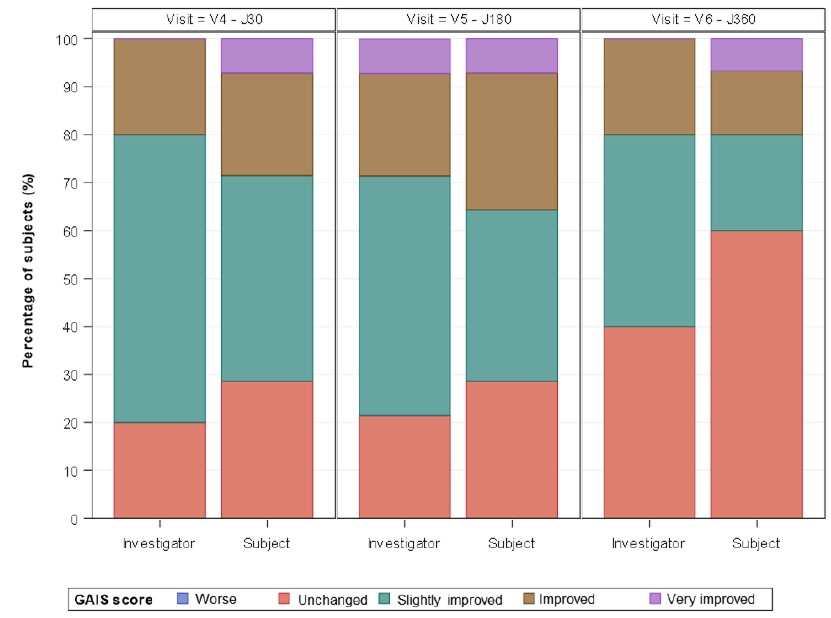
Material and methods: The study was performed on 23 patients. After a liposuction and fat proceed, SVF and the matrix were extracted and preconditioned following Ameacell® procedure, then injected into wrinkles. Results were scored for WSRS and GAIS scales at 6, 12 months after surgery.
Results: No serious adverse reactions occurred. Regarding efficiency, whatever the baseline, the Ameacell® procedure triggered an improvement of WSRS score in most patients after one year follow-up: 75% of patients when WSRS score at baseline 2; 83% of patients when WSRS score at baseline 3. This is in accordance with the GAIS scored by both investigators and patients.
Conclusion: This one-year follow-up clinical study using Ameacell® procedure, exhibited an improvement of facial skin appearance.
Downloads
References
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–95.
Rigotti G, Charles-de-Sá L, Gontijo-de-Amorim NF, Takiya CM, et al. Expanded Stem Cells, Stromal-Vascular Fraction, and Platelet-Rich Plasma Enriched Fat: Comparing Results of Different Facial Rejuvenation Approaches in a Clinical Trial. Aesthet Surg J 2016;36:261–70.
Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther 2010;5:103–10.
Bacakova L, Zarubova J, Travnickova M, Musilkova J, et al. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells – a review. Biotechnol Adv 2018;36:1111–1126.
Klar AS, Güven S, Biedermann T, Luginbühl J, et al. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 2014;35:5065–78.
Lee JH, Park J, Shin DW. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022;27:4351.
Klar AS, Michalak-Mićka K, Biedermann T, Simmen-Meuli C, et al. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr Surg Int 2018;34:129–135.
Estrada JC, Albo C, Benguría A, Dopazo A, et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death Differ 2012;19:743–55.
Dirat B, Samouillan V, Dandurand J, Gardou JP, et al. Positive effects of hypoxic preconditioning of matrix and stromal vascular fraction from adipose tissue. JPRAS Open 38 (2023) 173–185.
Bertozzi N, Simonacci F, Grieco MP, Grignaffini E, et al. The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Ann Med Surg (Lond) 2017;20:41–48.
Domenis R, Lazzaro L, Calabrese S, Mangoni D, et al. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther 2015;6:2.
Surowiecka A, Stru¿yna J. Adipose-Derived Stem Cells for Facial Rejuvenation. J Pers Med 2022;12:117.
Laloze J, Lupon E, Usseglio J, Girard P, et al. Measured Level of Human Adipose Tissue-Derived Stem Cells in Adipose Tissue is Strongly Dependent on Harvesting Method and Stem Cell Isolation Technique. Plast Reconstr Surg 2021;147:346e–347e.
Nebel S, Lux M, Kuth S, Bider F, et al. Alginate Core-Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering (Basel) 2022;9:66.
Pattayadeekul T, Pawcsuntorn T, Nararatwanchai T. The Efficacy and Safety of Autologous Stromal Vascular Fraction Transplantation for Infraorbital Skin Rejuvenation: A Clinical Prospective Study 2021. J Cosmet Dermatol 2022; 21:220–226.
Danesh A, Inglis HC, Jackman RP, Wu S, et al. Exosomes from Red Blood Cell Units Bind to Monocytes and Induce Proinflammatory Cytokines, Boosting T-Cell Responses in Vitro. Blood 2014, 123: 687–96.
Bowles AC, Wise RM, Gerstein BY, Thomas RC, et al. Immunomodulatory Effects of Adipose Stromal Vascular Fraction Cells Promote Alternative Activation Macrophages to Repair Tissue Damage. Stem Cells 2017;35:2198–2207.
Peinado JR, Jimenez-Gomez Y, Pulido MR, Ortega-Bellido M, et al. The stromal-vascular fraction of adipose tissue contributes to major differences between subcutaneous and visceral fat depots. Proteomics 2010;10:3356–66.
Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res 2008;332:415–26.
Charles-de-Sá L, Gontijo-de-Amorim NF, Maeda Takiya C, Borojevic R, et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg 2015;135:999–1009.
Kilinc MO, Santidrian A, Minev I, Toth T, et al. The ratio of ADSCs to HSC-progenitors in adipose tissue derived SVF may provide the key to predict the outcome of stem-cell therapy. Clin Transl Med 2018; 71:5.
Liu K, Cai J, Li H, Feng J, et al. The Disturbed Function of Neutrophils at the Early Stage of Fat Grafting Impairs Long-Term Fat Graft Retention. Plast Reconstr Surg 2018; 142:1229–1238.
Cai J, Feng J, Liu K, Zhou S, et al. Early Macrophage Infiltration Improves Fat Graft Survival by Inducing Angiogenesis and Hematopoietic Stem Cell Recruitment. Plast Reconstr Surg 2018;141:376–386.
Cai W, Yu LD, Tang X, Shen G. The Stromal Vascular Fraction Improves Maintenance of the Fat Graft Volume: A Systematic Review. Ann Plast Surg 2018;81:367–371.
Chung HM, Won CH, Sung JH. Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential. Expert Opin Biol Ther 2009; 9:1499–508.
Choi JR, Yong KW, Wan Safwani WKZ. Effect of hypoxia on human adipose-derived mesenchymal stem cells and its potential clinical applications. Cell Mol Life Sci. 2017;74:2587–2600.