Plant Medicine, Psychedelics and the Inner-Sciences of Mental Wellness: Implications for Healthy Longevity and Rejuvenation
DOI:
https://doi.org/10.13052/ijts2246-8765.2024.022Keywords:
Ayahuasca, Rythmia, Plant Medicine, Costa RicaAbstract
Rythmia Life Advancement Center is the first and currently only medically licensed Ayahuasca center in the world. Rythmia is helping to bridge the gap between the Western medical model of health care with the indigenous healing modalities of Latin America. The “Rythmia Way” program uses a variety of modalities, in addition to Ayahuasca, to help individuals find meaning in their lives and overcome depression, anxiety, trauma and PTSD. The quickly growing field of psychedelic medicine is in need of a format to facilitate the use of these substances in the most effective manner. The rapid rate of healing can pose challenges for traditional providers, which is why Rythmia is at the forefront of this field and is collecting hundreds of thousands of data points to effectively tailor and manage a safe plant medicine experience. Self-reported patient data, combined with observational program participation is collected and evaluated to enable individuals to achieve the most effective outcomes regarding their goals and intentions.
Mental health issues are becoming the number one health concern in the post-pandemic world. Depression and anxiety are preventing millions from living a productive life. Mental health issues are the leading cause of suicide globally. The information collected at Rythmia Life Advancement Center in Costa Rica shows a programmatic strategy that is effective at reducing depression, anxiety, and symptoms related to trauma, based on self-reported participant exit surveys. The traditional routes for individuals suffering from these mental health conditions (psychotropic medication, therapy, hospitalization, etc.), are not producing the outcome results necessary for society to thrive. Rythmia L.A.C. is providing a blueprint for the healthcare industry to follow which addresses these problems in a quick and efficient manner.
Rythmia Life Advancement Center is a licensed medical facility from the Costa Rican Ministry of Health, founded by Gerard Powell, and located in the Blue Zone Province of Guanacaste, Costa Rica. Mr. Powell was a self-made multimillionaire who had developed a severe drug and alcohol problem and lived a self-destructive lifestyle up until the age of 50. After 2 failed suicide attempts, an unsuccessful 60-day inpatient stay at a drug rehab, and 5 years of therapy riddled with relapse and depression, Mr. Powell discovered Ayahuasca as a treatment modality that showed benefit to mitigate his mental health issues. The lifechanging impact of Ayahuasca on Mr. Powell’s life was transformational and took place over a very short period of time. Mr. Powell was so enthusiastic about his profound level of healing that he wanted to share what he experienced with the world. In July of 2014, he enlisted the help of his therapist, Dr. Jeff McNairy, to participate in the Ayahuasca experience, and to help establish the first and only medically licensed Ayahuasca center in the world. Upon receiving Dr. McNairy’s personal breakthroughs with the plant medicine, he made the decision to shift his career from health care management of drug and alcohol rehabilitation and his therapy practice in Los Angeles, California, to help create the first of its kind wellness center based around Ayahuasca. Since Rythmia’s opening in January 2016, over 15,000 guests have participated in the program with a 97% success rate, determined by exit survey data gathered upon discharge.
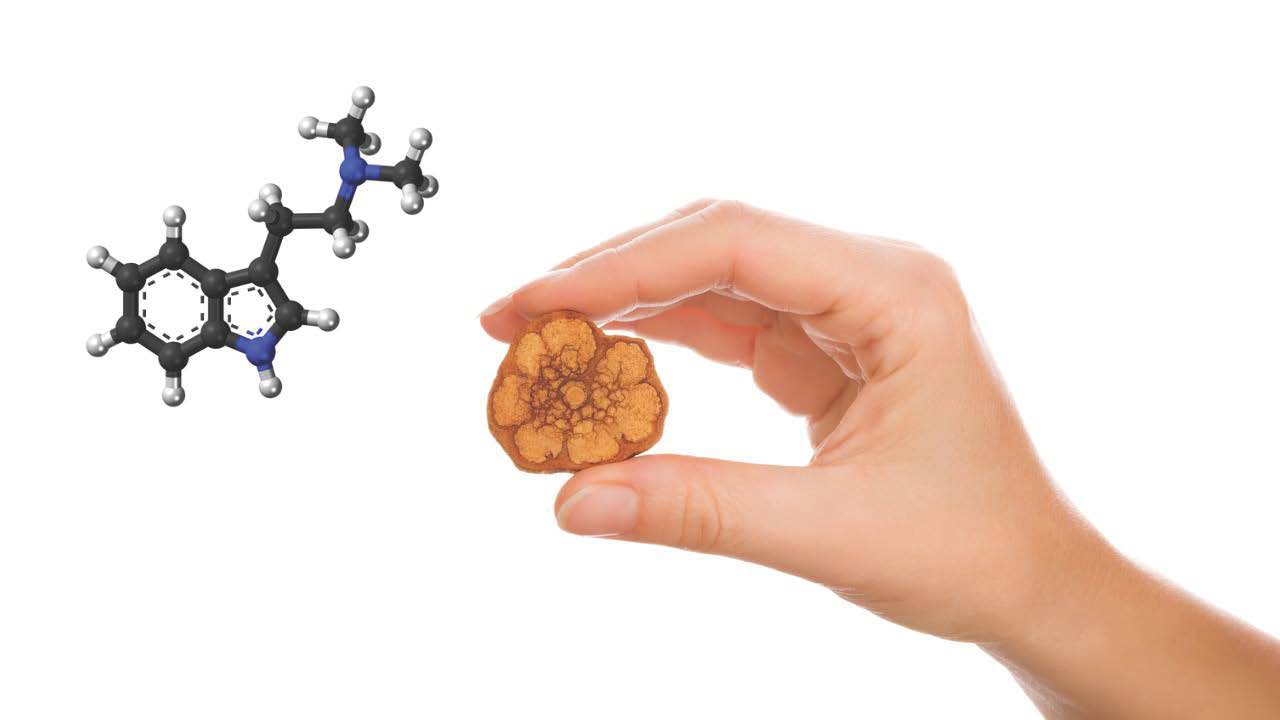
Ayahuasca is an indigenous plant-based medicinal drink, originating from the Amazon basin of South America. Shamans from this region of Latin America have been using this remedy for over three thousand years. Indigenous tribes consume Ayahuasca as a method to heal physical and “spiritual” ailments. The drink is made up of two plants: one is a vine known as Banisteriopsis caapi which contains three important alkaloids (harmine, harmaline, and tetrahydroharmine) which are Monoamine Oxidase-Inhibitors (MAO-I’s). The second plant is a N,N-Dimethyltryptamine (DMT) rich leaf-based plant named Psychotria viridis, which is found regionally where the shamanic tribes originate. When these two plants are reduced, mixed together, and consumed orally, they produce a psychedelic response in humans that creates a serotonin response said to enhance personal connection with self, nature, and others. The peak reaction point of the Ayahuasca is approximately 2 hours, with a half-life of DMT being anywhere from 2.5 to 4 hours. The contraindication risk for Ayahuasca is primarily with SSRI medications, which interact with MAO-I’s to potentially produce a serotonin syndrome in the brain. Participants must have at least 30 days free and clear from SSRI’s before drinking Ayhuasca, as well as be free of illicit drugs, alcohol, and other psychtropic medications. A certain amount of preparation is required prior to the consumption of Ayahuasca, in order to ensure a safe and effective experience.
Rythmia has established an admission protocol in which users must undergo multiple screenings before entering the program. The aim is to prevent any complications following ayahuasca ingestion. This is why there is a policy of 30 days without the use of any substance that affects certain neurochemical receptors and neurotransmitters. Additionally, applicants should not have any cardiac conditions, and on a psychological level, the absence of psychiatric illnesses such as bipolar disorder, schizophrenia, or prior episodes of psychosis is required. As a result of participation in the program, users have reported the resolution of psychiatric conditions such as depression, anxiety, post-traumatic stress, as well as a reduced need for pharmaceutical support upon returning home. Finding a greater connection with loved ones and overall life satisfaction is also reported by participants from post-program follow-up.
The “Rythmia Way” is a program based on specific directives from the CEO and Owner of Rythmia (Gerard Powell) that allow participants to maximize their experience with Ayahuasca. The program is comprised of various classes, Ayahuasca sessions, and workshops that teach three major intentions guests use to obtain their goals: 1. Show Me Who I’ve Become, 2. Merge Me Back With My Soul At All Costs, and 3. Heal My Heart. These intentions, when followed, will empower the individual to learn about their ego, no longer live in a dissociated state, and heal past traumas that are blocking them from being vulnerable in healthy ways and succeeding in life. Rythmia is a data driven program that looks at process and outcome data to fine tune and create a way of obtaining the highest success rates. The combination of informational classes and integration workshops helps provide guidance and understanding to the plant medicine experience.
As global jurisdictions begin to legally allow Ayahuasca as a legitimate health modality to be used under strict supervision and control, Rythmia Life Advancement Center is positioned to expand and create further opportunities for individuals to obtain significant and long-lasting healing. DMT is currently a Schedule 1 substance that is banned in most of the world. Certain exemptions are beginning to be made for research, communities of high risk and minimal resources, and as an alternative method for healing that is being re-evaluated since the UN Convention on Psychotropic Substances of 1971. As education and grass-roots organizations make headway in the areas of legalization, standards of care will need to be instituted to ensure safety, efficacy, and lasting duration of the process. Rythmia has set the standard of care in this field and will continue to lead the world as governments and health care agencies understand the benefits of these organic and ancient plants that have been combined thousands of years ago for the good of humanity as well as the earth. This commentary described an early clinical treatment that would require controlled clinical trial to transition as standard of care.
Downloads