Stromal Carcinoma Associated Fibroblasts Promote Drug Resistance of Human Pancreatic Cancer Cells by Modulation of ROS via CXCR4/CXCL12 Signaling
DOI:
https://doi.org/10.13052/ijts2246-8765.2015.006Abstract
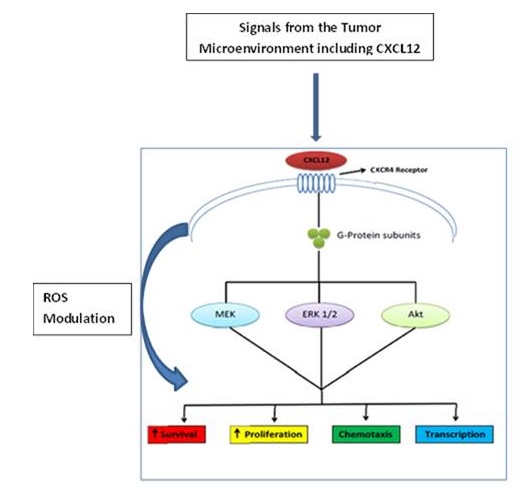
Pancreatic cancer is one of the most aggressive malignancies, with a 5-year overall survival of less than 5%. Tumor drug resistance to conventional chemotherapy, such as Gemcitabine, is often a significant contributor to poor overall survival. One of the common mechanisms of Gemcitabine resistance is activation of cell signaling via increased phosphorylation of mitogen-Activated kinase (MAP) kinases, leading to increased tumor sur- vival and reduced sensitivity to chemotherapeutic agents. A growing body of evidence suggests that the CXCL12/CXCR4 signal transduction axis in the tumor microenvironment is an important mediator of tumor migration, growth, and drug resistance. We hypothesized that stromal cells such as carcinoma-associated fibroblasts (CAFs), an important cellular component of the tumor microenvironment (TME), play a contributory role in the growth, invasiveness, and drug response of pancreatic cancer cells (PCCs) by activating CXCL12/CXCR4-mediated signal transduction. To test this, we used an in vitro model system to study the growth, invasion, and drug response of human PCCs in the presence or absence of in vitro generated CAFs and their precursors, the mesenchymal stem cells (MSCs). Functional studies demonstrated that co-culture of PCCs with CAFs led to signifi- cant increase in tumor cell invasion, which was abrogated by blockade of CXCR4 by plerixafor (AMD3100), a CXCR4 antagonist, and by siRNA- mediated knockdown of CXCR4 in CAFs. Further, we examined the effect of AMD3100 on the chemoresistance of PCCs to Gemcitabine. Our results indicated that AMD3100 reversed Gemcitabine-mediated chemoresistance of PCCs cells in the presence of CAFs or CAFconditioned media. In co- culture with pancreatic cancer cells, CAFs induced activation of MAPK signaling pathways and enhanced transcription of Mn-superoxide dismutase (SOD), glucose 6-phosphate dehydrogenase (G6PD), and catalase, genes involved in reactive oxygen species (ROS) pathways. Using Phloretin (a natural flavonoid found in apple leaves), a ROS inhibitor, we observed attenuation of MAPK signaling, SOD activity, and reversal of drug resistance in CAF-exposed pancreatic cancer cells. Phloretin in combination with Gem- citabine produced remarkable antitumor effects in a tumor xenograft model in immune compromised animals. These findings implicate CXCL12/CXCR4- dependent MAPK signaling and ROS pathways in CAF-mediated modulation of the growth, migration, and drug resistance of PCCs and provide a rationale for developing combination therapies for control of pancreatic cancer.
Downloads
References
Thota, R., J. M. Pauff, and J. D., Berlin. 2014. Treatment of metastatic
pancreatic adenocarcinoma: a review. Oncology (Williston Park) 28 (1):
–74. PubMed PMID:24683721.
Domanska, U. M., R. C. Kruizinga, W. B. Nagengast, H. Timmer-
Bosscha, G. Huls, E. G. de Vries, and A. M. Walenkamp. 2013. A review
on CXCR4/CXCL12 axis in oncology: no place to hide (Review).
Eur. J. Cancer 49 (1): 219–230. doi: 10.1016/j.ejca.2012.05.005.
PubMed PMID:22683307.
Statistics and outlook for pancreatic cancer. 2013. Cancer Research UK
www.cancerresearchuk.org/cancer.../pancreatic-canc.
Powis M. E., and K. J. Chang. 2000. Endoscopic ultrasound in the
clinical staging and management of pancreatic cancer: its impact on
cost of treatment (Review). Cancer Contr. 7 (5): 413–420. PubMed
PMID:11000610.
Pancreatic Cancer Action. NCIN Cancer Prevalence Atlas http://www.
ncin.org.uk/Prevalence/1 5 10 Year/atlas.html, 31/07/2012.
The Pancreatic Cancer Action Network Report 2012 (The alarming rise
of pancreatic cancer deaths in The United States: Why we need to stem
The Tide Today) www.pancan.org
Medscape. 2014. Surgery is the primary mode of treatment for
pancreatic cancer. However, an important role exists for chemother-
apy and/or radiation therapy. emedicine.medscape.com/article/280605-
overview.
Donadelli, M., I. Dando, T. Zaniboni, C. Costanzo, E. DallaPozza,
M. T. Scupoli, A. Scarpa, S. Zappavigna, M. Marra, A. Abbruzzese,
M. Bifulco, M. Caraglia, and M. Palmieri. 2011. Gemcitabine/
cannabinoid combination triggers autophagy in pancreatic cancer
cells through a ROS-mediated mechanism. Cell Death Dis. 2 (4): e152.
doi: 10.1038/cddis.2011.36. PMCID:PMC3122066.
Diehn, M., R. W. Cho, N. A. Lobo, T. Kalisky, M. J. Dorie, A. N. Kulp,
D. Qian, J. S. Lam, L. E. Ailles, M. Wong, B. Joshua, M. J. Kaplan,
I. Wapnir, F. M. Dirbas, G. Somlo, C. Garberoglio, B. Paz, J. Shen,
S. K. Lau, S. R. Quake, J. M. Brown, I. L. Weissman, and
M. F. Clarke. 2009. Association of reactive oxygen species levels
and radioresistance in cancer stem cells. Nature 458 (7239): 780–783.
doi: 10.1038/nature07733. PubMed PMID:19194462; PubMed Central
PMCID:PMC2778612.
Mini, E., S. Nobili, B. Caciagli, I. Landini, and T. Mazzei. 2006. Cellular
pharmacology of Gemcitabine (Review). Ann. Oncol. 17 (Suppl 5):
v7–v12. PubMed PMID:16807468.
Arora, S., A. Bhardwaj, S. Singh, S. K. Srivastava, S. McClellan,
C. S. Nirodi, G. A. Piazza, W. E. Grizzle, L. B. Owen, and A. P. Singh.
An undesired effect of chemotherapy: gemcitabine promotes
pancreatic cancer cell invasiveness through reactive oxygen species-
dependent, nuclear factor κB- and hypoxia-inducible factor 1α-mediated
up-regulation of CXCR4. J. Biol. Chem. 288 (29): 21197–21207.
doi:10.1074/jbc.M113.484576. PubMed PMID: 23740244; PubMed
Central PMCID:PMC3774385.
American Chemical Society, Washington, DC. 1995. Polyphenolic pat-
tern in apple tree leaves in relation to scab resistance. A preliminary
study. J. Agric. Food Chem. 43 (8): 2273–2278 ISSN 0021-8561
CODENJAFCAU.
Rezk, B. M., G. R. Haenen, W. J. van der Vijgh, and A. Bast. 2002.
The antioxidant activity of Phloretin: the disclosure of a new antioxidant
pharmacophore in flavonoids. Biochem. Biophys. Res. Commun. 295 (1):
–13. PubMed PMID:12083758.
Bergman, M., A. Perelman, Z. Dubinsky, and S. Grossman. 2003.
Scavenging of reactive oxygen species by a novel glucurinated flavonoid
antioxidant isolated and purified from spinach. Phytochemistry 62 (5):
–762. PubMed PMID:12620328.
Mahadevan, D., and D. D. Von Hoff. 2007. Tumor–stroma interactions
in pancreatic ductal Adenocarcinoma (Review). Mol. Cancer Ther.
(4): 1186–1197. PubMed PMID:17406031.
Houthuijzen, J. M., L. G. Daenen, J. M. Roodhart, and E. E. Voest.
The role of mesenchymal stem cells in anti-cancer drug resistance
and tumour progression (Review). Br. J. Cancer 106 (12): 1901–1906.
doi: 10.1038/bjc.2012.201. PubMed PMID: 22596239; PubMed Central
PMCID: PMC3388567.
Mishra, P., D. Banerjee, A., and Ben-Baruch. 2011. Chemokines at the
crossroads of tumor-fibroblast interactions that promote malignancy
(Review). J. Leukoc. Biol. 89 (1): 31–39. doi: 10.1189/jlb.0310182.
PubMed PMID:20628066.
Bhagat, T. D., Y. Rattigan, B. Patel,·S. Mirte,·Y. Yu, D. Sohal, et al.
Pancreatic cancer associated fibroblasts are characterized by
widespread epigenetic reprogramming that leads to aberrant expression
of druggable target CXCR4. Abstract Number: 1504 AACR Annual
Meeting Washington DC.
Macanas-Pirard, P., Leisewitz A, Broekhuizen R, Cautivo K,
Barriga FM, Leisewitz F, Gidi V, Riquelme E, Montecinos VP,
Swett P, Besa P, Ramirez P, Ocqueteau M, Kalergis AM, Holt M,
Rettig M, DiPersio JF, Nervi B. Bone marrow stromal cells modulate
mouse ENT1 activity and protect leukemia cells from cytarabine induced
apoptosis. PLoS One. 2012; 7 (5): e37203. doi: 10.1371/journal.pone.
PubMed PMID: 22629369; PubMed Central PMCID:
PMC3358339.
Gao, H., W. Priebe, J. Glod, D. Banerjee. 2009. Activation of signal
transducers and activators of transcription 3 and focal adhesion kinase
by stromal cell-derived factor 1 is required for migration of human mes-
enchymal stem cells in response to tumor cell-conditioned medium. Stem
Cells 27 (4): 857–865. doi: 10.1002/stem.23. PubMed PMID:19350687.
Teicher, B. A., and S. P. Fricker. 2010. CXCL12 (SDF-1)/CXCR4
pathway in cancer (Review). Clin. Cancer Res. 16 (11): 2927–2931.
doi: 10.1158/1078-0432.CCR-09-2329. PubMed PMID:20484021.
Macanas-Pirard, P., A. Leisewitz, R. Broekhuizen, K. Cautivo, F. M.
Barriga, F. Leisewitz, V. Gidi, E. Riquelme, V. P. Montecinos, P. Swett,
P. Besa, P. Ramirez, M. Ocqueteau, A. M. Kalergis, M. Holt, M. Rettig,
J. F. DiPersio, B. Nervi. 2012 Bone marrow stromal cells modulate
mouse ENT1 activity and protect leukemia cells from cytarabine induced
apoptosis. PLoS One. 7 (5): e37203. doi: 10.1371/journal.pone.0037203.
PubMed PMID:22629369; PubMed Central PMCID:PMC3358339.
Turrens, J. F. 2003. Mitochondrial formation of reactive oxygen species
(Review). J. Physiol. 552 (Pt 2): 335–344. PubMed PMID:14561818;
PubMed Central PMCID:PMC2343396.
Wagner, B. A., G. R. Buettner, and C. P. Burns. 1994. Free radical-
mediated lipid peroxidation in cells: oxidizability is a function of cell
lipid bis-allylic hydrogen content. Biochemistry 33 (15): 4449–4453.
PubMed PMID:8161499.
Chetram, M. A., C. V. Hinton. 2013. ROS-mediated regula-
tion of CXCR4 in cancer. Front. Biol. (Beijing) 8 (3). doi:
1007/s11515-012-1204-4. PubMed PMID:24223583; PubMed Cen-
tral PMCID:PMC3820291.
Middleton, G., P. Ghaneh, E. Costello, W. Greenhalf, and
J. P. Neoptolemos. 2008. New treatment options for advanced pan-
creatic cancer (Review). Expert Rev. GastroenterolHepatol. 2 (5):
–696. doi: 10.1586/17474124.2.5.673. PubMed PMID:19072345.
Ishida T., A. Nakaizumi, S. Tanaka, and M. Tatsuta. 2006. Early detec-
tion of pancreatic cancer (Review, Japanese). 64 (Suppl. 1): 155–159.
PubMed PMID:16457240.
Yokoi, K., and I. J. Fidler. 2004. Hypoxia increases resistance of human
pancreatic cancer cells to apoptosis induced by Gemcitabine. Clin.
Cancer Res. 10 (7): 2299–2306. PubMed PMID:15073105.
Singh, A. P., S. Arora, A. Bhardwaj, S. K. Srivastava, M. P. Kadakia,
B. Wang, W. E. Grizzle, L.B. Owen, S. Singh. 2012. CXCL12/CXCR4
protein signaling axis induces sonic hedgehog in pancreatic can-
cer cells via extracellular regulated kinase- and Akt kinase-mediated
activation of nuclear factor κB: implications for bidirectional tumor–
stromal interactions. J. Biol. Chem. 287 (46): 39115–39124. doi:
1074/jbc.M112.409581. PubMed PMID: 22995914; PubMed Central
PMCID: PMC3493952.
Chetram, M. A., and C. V. Hinton. 2013. ROS-mediated regulation of
CXCR4 in cancer. Front. Biol. (Beijing) 8 (3). doi: 10.1007/s11515-
-1204-4. PubMed PMID:24223583; PubMed Central PMCID:
PMC3820291.
Trédan, O., C. M. Galmarini, K Patel, I. F. Tannock 2007. Drug resistance
and the solid tumor microenvironment. J. Natl. Cancer Inst. 99 (19):
–1454. Review. PubMed PMID:17895480.
Mishra, P. J., R. Humeniuk, D. J. Medina, G. Alexe, J. P. Mesirov,
S. Ganesan, J. W. Glod, D. Banerjee. 2008. Carcinoma-associated
fibroblast-like differentiation of human mesenchymal stem cells.
Cancer Res. 68 (11): 4331–4339. doi: 10.1158/0008-5472.CAN-
-0943. PubMed PMID:18519693; PubMed Central PMCID:
PMC2725025.