Cryopreservation of Large Number of Human Hematopoietic Cells – A Model for Research Space with Limited Resources
DOI:
https://doi.org/10.13052/ijts2246-8765.2024.002Keywords:
Cell storage, cryopreservation, mobilized peripheral blood, transplant, stem cell, hematopoiesisAbstract
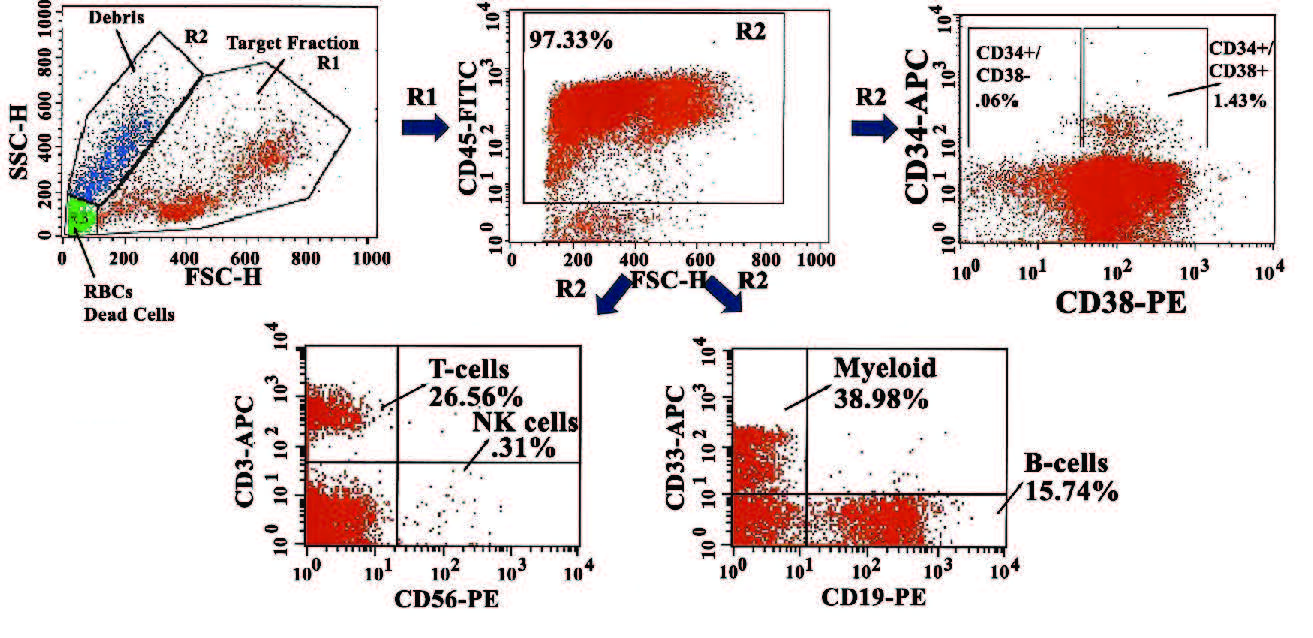
Unlike cell lines, cells from primary human tissues are valuable, requiring storage for long-term use. Large number of primary cells require the most efficient method of shipment, when relevant, and cryopreservation to preserve the viability and function. Shipping of cells could be determined by the specific experimental question. These issues are particularly important for primary cells such as hematopoietic stem cells, which cannot expand in vitro. There is minimum issues when cryopreserving relatively small number of cells from tissues such as umbilical cord blood and bone marrow aspirates. However, cryopreservation of >200 × 109 primary cells comes with challenges to identify the most efficient method for long-term storage. Here we report on processes to achieve efficient cell health and recovery of hematopoietic cells from mobilized peripheral blood cells (MPBs). We also determined overnight shipment of MPBs led to overall outcomes when shipped in the cold, as compared to room temperature. We found that cryopreservation of 50 × 106 cells in 2 mL led to maximum recovery of hematopoietic cell subsets. The implication for these conditions to achieve efficient long-term storage is discussed. These methods will benefit small research laboratories such as those at academic settings and start-up companies with limited resources.
Downloads
References
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
Wei X, Yang X, Han Z-p, Qu F-f, Shao L, Shi Y-f. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacologica Sinica. 2013;34(6):747–54.
Thomas ED. Bone marrow transplantation: a historical review. Medicina. 2000;33(3):209–18.
Dreger P, Haferlach T, Eckstein V, Jacobs S, Suttorp M, Löuffler H, et al. G-CSF-mobilized peripheral blood progenitor cells for allogeneic transplantation: safety, kinetics of mobilization, and composition of the graft. Br J Haematol. 1994;87(3):609–13.
Treleaven JG, Mehta J. Bone marrow and peripheral blood stem cell harvesting. J Hematother. 1992;1(3):215–23.
6. Greco SJ, Ayer S, Guiro K, Sinha G, Donnelly RJ, El-Far MH, et al. Restoration of aged hematopoietic cells by their young counterparts through instructive microvesicles release. Aging (Albany NY). 2021;13(21):23981–4016.
Bender JG, Unverzagt K, Walker DE, Lee W, Smith S, Williams S, et al. Phenotypic analysis and characterization of CD34+
cells from normal human bone marrow, cord blood, peripheral blood, and mobilized peripheral blood from patients undergoing autologous stem cell transplantation. Clin Immunol Immunopathol. 1994;70(1):10–8.
Gergues M, Ayer S, Morelli S, Greco SJ, Rameshwar P. Hematological Humanization of Immune-Deficient Mice. Methods Mol Biol. 2021;2224:195–202.
Romagano MP, Sherman LS, Shadpoor B, El-Far M, Souayah S, Pamarthi SH, et al. Aspirin-Mediated Reset of Preeclamptic Placental Stem Cell Transcriptome – Implication for Stabilized Placental Function. Stem Cell Rev Rep. 2022;18(8):3066–82.
Sherman LS, Condé-Green A, Naaldijk Y, Lee ES, Rameshwar P. An Enzyme-free Method for Isolation and Expansion of Human Adipose-derived Mesenchymal Stem Cells. J Vis Exp. 2019(154).
Sherman LS, Shaker M, Mariotti V, Rameshwar P. Mesenchymal stromal/stem cells in drug therapy: New perspective. Cytotherapy. 2017;19(1):19–27.