Restore Veto Property in Low Dose Aspirin/ASA Treated Preeclampsia Placenta Mesenchymal Stem Cells: Insights Into ASA-mediated Clinical Response*
DOI:
https://doi.org/10.13052/ijts2246-8765.2024.025Abstract
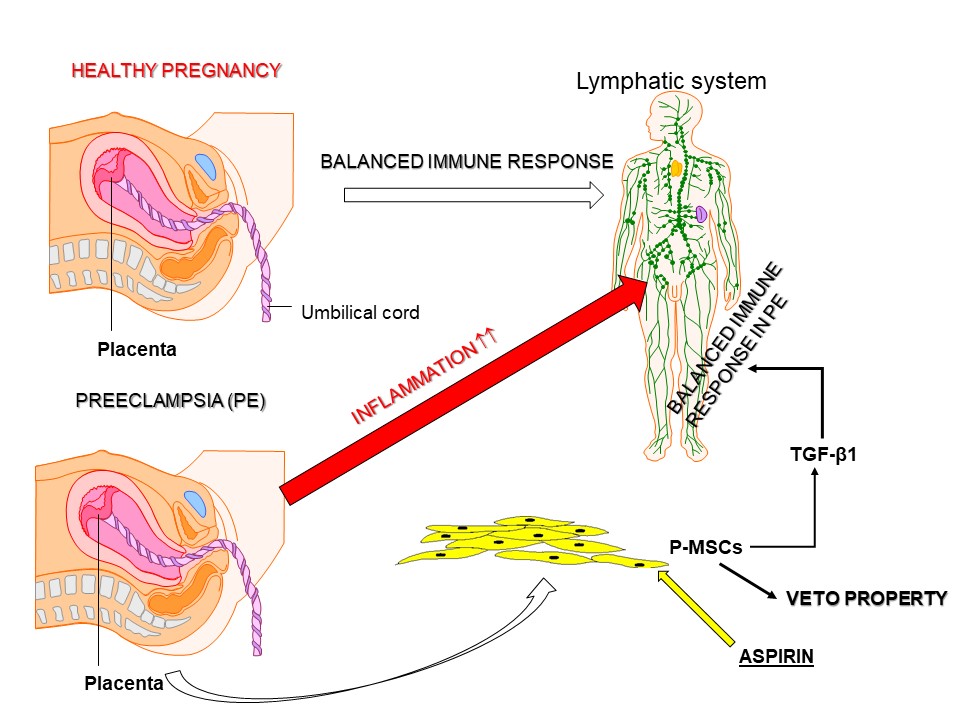
Preeclampsia (PE) is a pregnancy complication characterized by elevated blood pressure, proteinuria, and other laboratory abnormalities. PE affects 2–8% of pregnancies globally and can lead to preterm birth and other complications for the mother and fetus. Successful pregnancy depends on the ability of the mother’s immune system to tolerate the allogeneic fetus. However, in PE, this immune tolerance is exacerbated by inflammation. Transforming growth factor β (TGF-β) is important to retain an immune balance in healthy pregnancy. In PE, TGF-β level is reduced, with an imbalance of the T-cell subset pool to favor inflammation. Omics studies by our group reported an increase in TGF-β signaling when PE-derived placenta mesenchymal stem cells (P-MSCs) were treated with low dose aspirin (ASA). This correlated with increased cycling quiescence and epigenetic changes, resembling healthy P-MSCs. This study tested the hypothesis that ASA could restore the veto property of P-MSCs to mitigate inflammation. ASA (10 mM) treated P-MSCs from PE and healthy placentas increased TGFβ1 and its receptor. The ASA treated MSCs, when added as third-party cells to a one-way mixed lymphocyte reaction, suppressed T-cell proliferation. Prediction studies with omics data indicated that ASA-mediated TGFβ signaling could explain ASA-induced blunting of cell apoptosis. Together, the findings support ASA-mediated expression of TGFβ1 and its receptor on P-MSCs from PE to restore the ability to be licensed as immune suppressor to mitigate PE inflammation. The findings provide new insight into the benefit of ASA treatment for PE.
Downloads
References
Force USPST, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: US Preventive Services Task Force Recommendation Statement. J Am Med Assoc. 2021;326:1186–1191.
Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–306.
Brooks SA, Martin E, Smeester L, Grace MR, Boggess K, Fry RC. miRNAs as common regulators of the transforming growth factor (TGF)-beta pathway in the preeclamptic placenta and cadmium-treated trophoblasts: Links between the environment, the epigenome and preeclampsia. Food Chem Toxicol. 2016;98(Pt A):50–7.
Wang Y, Guo X, Obore N, Ding H, Wu C, Yu H. Aspirin for the prevention of preeclampsia: A systematic review and meta-analysis of randomized controlled studies. Front Cardiovasc Med. 2022;9:936560.
Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Aspirin use to prevent preeclampsia and related morbidity and mortality: US Preventive Services Task Force recommendation statement. J Am Med Assoc. 2021;326:1186–91.
Cornelius DC. Preeclampsia: From Inflammation to Immunoregulation. Clin Med Insights Blood Disord. 2018;11:1179545X17752325.
Tsakiridis I, Giouleka S, Arvanitaki A, Giannakoulas G, Papazisis G, Mamopoulos A, et al. Gestational Hypertension and Preeclampsia: An Overview of National and International Guidelines. Obstet Gynecol Surv. 2021;76:613–633.
Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet Gynecol. 2020;135:1492–1495.
Simpson H, Robson SC, Bulmer JN, Barber A, Lyall F. Transforming growth factor beta expression in human placenta and placental bed during early pregnancy. Placenta. 2002;23:44–58.
Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179:1359–1375.
Jena MK, Sharma NR, Petitt M, Maulik D, Nayak NR. Pathogenesis of preeclampsia and therapeutic approaches targeting the placenta. Biomolecules. 2020;10:953.
Atallah A, Lecarpentier E, Goffinet F, Doret-Dion M, Gaucherand P, Tsatsaris V. Aspirin for Prevention of Preeclampsia. Drugs. 2017;77:1819–1831.
ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet Gynecol. 2018;132:e44–e52.
Romagano MP, Sherman LS, Shadpoor B, El-Far M, Souayah S, Pamarthi SH, et al. Aspirin-Mediated Reset of Preeclamptic Placental Stem Cell Transcriptome – Implication for Stabilized Placental Function. Stem Cell Rev Rep. 2022;18:3066–3082.
Abu-Raya B, Michalski C, Sadarangani M, Lavoie PM. Maternal Immunological Adaptation During Normal Pregnancy. Front Immunol. 2020;11:575197.
Zhang YJ, Shen L, Zhang T, Muyayalo KP, Luo J, Mor G, et al. Immunologic Memory in Pregnancy: Focusing on Memory Regulatory T Cells. Int J Biol Sci. 2022;18:2406–2418.
Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front Immunol. 2020;11:2025.
Warning JC, McCracken SA, Morris JM. A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction. 2011;141:715–724.
Robertson SA, Moldenhauer LM. Immunological determinants of implantation success. International J Dev Biol. 2014;58:205–217.
Kaicker A. Immune cells at the maternal-fetal interphase: Role in implantation and establishment of tolerance. Journal of Applied Biology and Biotechnology. 2023;11:45–50.
Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW, Jr., Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond). 2016;130:409–19.
Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-beta – an excellent servant but a bad master. J Transl Med. 2012;10:183.
Zhang Y, Alexander PB, Wang XF. TGF-beta Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb Perspect Biol. 2017;9:a022145.
Yang D, Dai F, Yuan M, Zheng Y, Liu S, Deng Z, et al. Role of Transforming Growth Factor-beta1 in Regulating Fetal-Maternal Immune Tolerance in Normal and Pathological Pregnancy. Front Immunol. 2021;12:689181.
Sanjabi S, Oh SA, Li MO. Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection. Cold Spring Harb Perspect Biol. 2017;9:a022236.
Sherman LS, Shaker M, Mariotti V, Rameshwar P. Mesenchymal stromal/stem cells in drug therapy: New perspective. Cytotherapy. 2017;19:19–27.
Viswanathan S, Ciccocioppo R, Galipeau J, Krampera M, Le Blanc K, Martin I, et al. Consensus International Council for Commonality in Blood Banking Automation-International Society for Cell & Gene Therapy statement on standard nomenclature abbreviations for the tissue of origin of mesenchymal stromal cells. Cytotherapy. 2021;23:1060–1063.
Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36: 568–584.
Helmy KY, Patel SA, Silverio K, Pliner L, Rameshwar P. Stem cells and regenerative medicine: accomplishments to date and future promise. Ther Deliv. 2010;1:693–705.
Eljarrah A, Gergues M, Pobiarzyn PW, Sandiford OA, Rameshwar P. Therapeutic Potential of Mesenchymal Stem Cells in Immune-Mediated Diseases. Adv Exp Med Biol. 2019;1201:93–108.
Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434.
Kang HS, Habib M, Chan J, Abavana C, Potian JA, Ponzio NM, et al. A paradoxical role for IFN-gamma in the immune properties of mesenchymal stem cells during viral challenge. Exp Hematol. 2005;33: 796–803.
Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712.
Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018;2018: 3057624.
Salari V, Mengoni F, Del Gallo F, Bertini G, Fabene PF. The Anti-Inflammatory Properties of Mesenchymal Stem Cells in Epilepsy: Possible Treatments and Future Perspectives. Intl J Mol Sci. 2020;21:9683.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736.
Krishnamoorthy K, Sherman LS, Romagano MP, El Far M, Etchegaray JP, Williams SF, et al. Low dose acetyl salicylic acid (LDA) mediates epigenetic changes in preeclampsia placental mesenchymal stem cells similar to cells from healthy pregnancy. Placenta. 2023;137:49–58.
Rameshwar P, Narayanan R, Qian J, Denny TN, Colon C, Gascon P. NF-kappa B as a central mediator in the induction of TGF-beta in monocytes from patients with idiopathic myelofibrosis: an inflammatory response beyond the realm of homeostasis. J Immunol. 2000;165:2271–2277.
Sandiford OA, Donnelly RJ, El-Far MH, Burgmeyer LM, Sinha G, Pamarthi SH, et al. Mesenchymal Stem Cell-Secreted Extracellular Vesicles Instruct Stepwise Dedifferentiation of Breast Cancer Cells into Dormancy at the Bone Marrow Perivascular Region. Cancer Res. 2021;81:1567–1582.
Hao W, Shi S, Zhou S, Wang X, Nie S. Aspirin inhibits growth and enhances cardiomyocyte differentiation of bone marrow mesenchymal stem cells. Eur J Pharmacol. 2018;827:198–207.
Wang Y, Chen X, Zhu W, Zhang H, Hu S, Cong X. Growth inhibition of mesenchymal stem cells by aspirin: involvement of the WNT/beta-catenin signal pathway. Clin Exp Pharmacol Physiol. 2006;33:696–701.
Fan W, Li J, Chen J, Zhu L, Wang Y, Sun B, et al. Aspirin inhibits the proliferation of synovium-derived mesenchymal stem cells by arresting the cell cycle in the G0/G1 phase. Am J Transl Res. 2017;9:5056–5062.
Cao Y, Xiong J, Mei S, Wang F, Zhao Z, Wang S, et al. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res Ther. 2015;6:210.
Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184:5885–5894.
Rambaldi MP, Weiner E, Mecacci F, Bar J, Petraglia F. Immunomodulation and preeclampsia. Best practice Res Clin Obstetrics Gynaecol. 2019;60:87–96.
Ai-ris YC, Smith LA, Karumanchi SA. Review of the immune mechanisms of preeclampsia and the potential of immune modulating therapy. Human Immunol. 2021;82:362–370.
Molvarec A, Szarka A, Walentin S, Beko G, Karadi I, Prohaszka Z, et al. Serum leptin levels in relation to circulating cytokines, chemokines, adhesion molecules and angiogenic factors in normal pregnancy and preeclampsia. Reprod Biol Endocrinol. 2011;9:124.
Yu L, Kuang LY, He F, Du LL, Li QL, Sun W, et al. The Role and Molecular Mechanism of Long Nocoding RNA-MEG3 in the Pathogenesis of Preeclampsia. Reprod Sci. 2018;25:1619–1628.