Stem Cell Therapy for Brain Tumors
DOI:
https://doi.org/10.13052/ijts2246-8765.2015.005Abstract
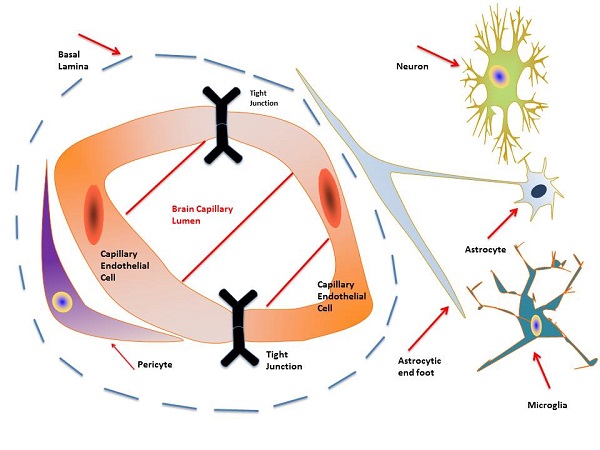
Glioblastoma multiforme (GBM), the most common and lethal brain cancer, prognosis remains bleak with a median survival of about 15 months despite maximal surgical resection, radiotherapy, and temozolomide treatment. The difficulty associated with safely and effectively delivering therapeutics across the blood brain barrier (BBB) is a major challenge towards GBM treatment. Ongoing research and clinical trials, including attempts to deliver therapeutics within stem cells present possible solutions. The relationships between brain cancer pathology, stem cell properties, therapeutic advantages and disadvan- tages of various stem cell types, drug delivery methods, cancer stem cells, gene therapy, anti-cancer vaccines, chimeric antigen receptor therapies, and combination therapies are discussed.
Downloads
References
Wen, P. Y., and S. Kesari. 2008. Malignant gliomas in adults. N Engl.
J. Med. 359: 492–507.
Lee, J. K., K. M. Joo, J. Lee, Y. Yoon, D. H. Nam. 2014. Targeting the
epithelial to mesenchymal transition in glioblastoma: the emerging role
of MET signaling. Onco Targets Ther. 7: 1933–1944.
Wilson, T. A., M. A. Karajannis, D. H. Harter. 2014. Glioblastoma
multiforme: State of the art and future therapeutics. Surg. Neurol. Int.
: 64.
Ferlay, J., H. R. Shin, F. Bray, D. Forman, C. Mathers, D. M. Parkin.
Estimates of worldwide burden of cancer in 2008: GLOBOCAN
Int. J. Cancer. 127: 2893–2917.
Serão, N. V. L., K. R. Delfino, B. R. Southey, J. E. Beever, and S.
L. Rodriguez-Zas. 2011. Cell cycle and aging, morphogenesis, and
response to stimuli genes are individualized biomarkers of glioblastoma
progression and survival. BMC Med. Genomics 4: 1–21
Dai, C., and E. Holland. 2005. Astrocyte differentiation states and
glioma formation. In Glioblastoma Multiforme. J. Markert, V. T. DeVita,
S. A. Rosenberg, and S. Hellman, eds. Jones and Barlett Publishers,
Subury, MA. p. 1–316.
Kleihues, P., and H. Ohgaki. Primary and secondary glioblastomas:
from concept to clinical diagnosis. Neuro-Oncology 1998: 44–51.
Morizane, A., J. Y. Li, P. Brundin. 2008. From bench to bedside: the
potential of stem cells for the treatment of Parkinson’s disease. Cell
Tissue Res. 331: 323–336.
Mouhieddine, T. H., F. H. Kobeissy, M. Itani, A. Nokkari, and K. K. W.
Wang. 2014. Stem cells in neuroinjury and neurodegenerative disorders:
challenges and future neurotherapeutic prospects. Neural Regen. Res.
: 901–906.
Feng, Z., and F. Gao. 2012. Stem cell challenges in the treatment of
neurodegenerative disease. CNS Neurosci. Ther. 18: 142–148.
Kim, S. U., H. J. Lee, and Y. B. Kim. 2013. Neural stem cell-based
treatment for neurodegenerative diseases. Neuropathology 33:
–504.
Wang, S., H. Cheng, G. Dai, X. Wang, R. Hua, X. Liu, P. Wang, G.
Chen, W. Yue, Y. An. 2013. Umbilical cord mesenchymal stem cell
transplantation significantly improves neurological function in patients
with sequelae of traumatic brain injury. Brain Res. 1532: 76–84.
Achyut, B. R., N. R. S. Varma, A. S. Arbab. 2014. Application of
umbilical cord blood derived stem cells in diseases of the nervous
system. J. Stem Cell Res. Ther. 4: 202.
Ali, H., N. Bayatti, S. Lindsay, A. A. Dashti, F. Al-Mulla. 2013.
Directed differentiation of umbilical cord blood stem cells into cortical
GABAergic neurons. Acta Neurobiol. Exp. 73: 250–259.
Volarevic, V., N. Arsenijevic, M. L. Lukic, and M. Stojkovic. 2011.
Concise review: mesenchymal stem cell treatment of the complications
of diabetes mellitus. Stem Cells 29: 5–10.
Sigurjonsson, O. E., M. C. Perreault, T. Egeland, J. C. Glover. 2005.
Adult human hematopoietic stem cells produce neurons efficiently in
the regenerating chicken embryo spinal cord. Proc. Natl. Acad. Sci.
USA 102: 5227–5232.
Pang, Z. P., N. Yang, T. Vierbuchen, A. Ostermeier, D. R. Fuentes, T. Q.
Yang, A. Citri, V. Sebastiano, S. Marro, T. C. Sudhof, M. Wernig. 2011.
Induction of human neuronal cells by defined transcription factors.
Nature 476: 220–223
Liu, X., F. Li, E. A. Stubblefield, B. Blanchard, T. L. Richards, G. A.
Larson, Y. He, Q. Huang, A. C. Tan, D. Zhang, T. A. Benke, J. R.
Sladek, N. R. Zahniser, C. Y. Li. 2012. Direct reprogramming of human
fibroblasts into dopaminergic neuron-like cells. Cell Res. 22: 321–32.
Takahashi, K., and S. Yamanaka. 2006. Induction of pluripotent stem
cells from mouse embryonic and adult fibroblast cultures by defined
factors. Cell 126: 663–676.
Sivapatham, R., and X. Zeng. 2014. Generation and characterization
of patient-specific induced pluripotent stem cell for disease modeling.
Methods Mol. Biol. 2014: 1–20.
Streckfuss-Bomeke, K., F. Wolf, A. Azizian, M. Stauske, M. Tiburcy,
S. Wagner, D. Hubscher, R. Dressel, S. Chen, J. Jende, G. Wulf, V.
Lorenz, M. P. Schon, L. S. Maier, W. H. Zimmermann, G. Hasenfuss,
and K. Guan. 2013. Comparative study of human-induced pluripotent
stem cells derived from bone marrow cells, hair keratinocytes, and skin
fibroblasts. Eur. Heart J. 34: 2618–2629.
Sunberg, M., H. Bogetofte, T. Lawson, J. Jansson, G. Smith, A.
Astradsson, M. Moore, T. Osborn, O. Cooper, R. Spealman, P. Hallet, O.
Isacson. 2013. Improved cell therapy protocols for Parkinson’s disease
based on differentiation efficiency and safety of hESC-, hiPSC-, and
non-human primate iPSC-derived dopaminergic neurons. Stem Cells
: 1548–1562.
Buttery, P. C., and R. A. Barker. 2014. Treating Parkinson’s dis-
ease in the 21st century: Can stem cell transplantation compete? J.
Comparative Neurol. 522: 2802–2816.
Kim, D., C. H. Kim, J. I. Moon, Y. G. Chung, M. Y. Chang, B. S.
Han, S. Ko, E. Yang, K. Y. Cha, R. Lanza, and K. S. Kim. 2009.
Generation of human induced pluripotent stem cells by direct delivery
of reprogramming proteins. Cell Stem Cell 4: 472–476.
Hou, L. L., and T. Hong. 2012. Stem cells and neurodegenerative
diseases. CNS Neurosci. Ther. 51:287–294.
Soldner, F., D. Hockemeyer, C. Beard, Q. Gao, G.W. Bell, E.G. Cook,
G. Hargus, A. Blak, O. Cooper, M. Mitalipova, O. Isacson, and R.
Jaenisch. 2009. Parkinson’s disease patient-derived induced pluripotent
stem cells free of viral reprogramming factors. Cell 136: 964–977.
Huang, G. T.-J., S. Gronthos, and S. Shi. 2009. Mesenchymal stem cells
derived from dental tissues vs. those from other sources. J. Dent. Res.
: 792–806.
Caplan, A. I., and J. E. Dennis. 2006. Mesenchymal stem cells as trophic
mediators. J. Cell Biochem. 98: 1076–1084.
Phiney, D. G., and D. J. Prockop. 2007. Mesenchymal stem/ multipotent
stromal cells: the state of transdifferentiation and modes of tissue repair-
current views. Stem Cells 25: 2896–2902.
Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas,
J. D. Mosca, M. A. Moorman, D. W. Moorman, D. W. Simonetti, S.
Craig, and D. R. Marshak.1999. Multilineage potential of adult human
mesenchymal stem cells. Science 284: 143–147.
Asakura, A., M. Komaki, M. A. Rudnicki. 2001. Muscle satellite cells
are multipotential stem cells that exhibit myogenic, osteogenic, and
adipogenic differentiation. Differentiation 68: 245–253.
De Bari, C., F. Dell’Accio, P. Tylzanowski, F. P. Luyten. 2001. Multi-
potent mesenchymal stem cells from adult human synovial membrane.
Arthritis Rheum. 44: 1928–1942.
Akimoto, K., K. Kimura, M. Nagano, S. Takano, G.T. Salazar, T.
Yamashita, and O. Ohneda. 2013. Umbilical cord blood-derived mes-
enchymal stem cells inhibit, but adipose tissue-derived mesenchymal
stem cells promote, glioblastoma multiforme proliferation. Stem Cells
Dev 22: 1370–1386.
Nagano, M., K. Kimura, T. Yamashita, K. Ohneda, D. Nozawa, H.
Hamada, H. Yoshikawa, N. Ochiai, and O. Ohneda. 2010. Hypoxia
responsive mesenchymal stem cells derived from human umbili-
cal cord blood are effective for bone repair. Stem Cells Dev 19:
–1210.
Bieback, K., S. Kern, H. Klüter, and H. Eichler. 2004. Critical param-
eters for the isolation of mesenchymal stem cells from umbilical cord
blood. Stem Cells 22: 625–634.
Parolini, O., F. Alviano, G. P. Bagnara, G. Bilic, H. J. Buhring, M.
Evangelista, S. Hennerbichler, B. Liu, M. Magatti, N. Mao, T. Miki, F.
Marongiu, H. Nakajima, T. Nikaido, C. B. Portmann-Lanz, V. Sankar,
M. Soncini, G. Stadler, D. Surbek, T. A. Takahashi, H. Redl, N.
Sakuragawa, S. Wolbank, S. Zeisberger, A. Zisch, and S. C. Strom.
Concise review: isolation and characterization of cells from
human term placenta: outcome of the first international Workshop on
Placenta Derived Stem Cells. Stem Cells 26: 300–311.
Tran, T. C., K. Kimura, M. Nagano, T. Yamashita, K. Ohneda, H.
Sugimori, F. Sato, Y. Sakakibara, H. Hamada, H. Yoshikawa. S. N.
Hoang, and O. Ohneda. 2011. Identification of human placenta-derived mesenchymal stem cells involved in re-endothelialization. J. Cell.
Physiol. 226: 224–235.
De Coppi, P., G. Bartsch, M. M. Siddiqui, T. Xu, C. C. Santos, L. Perin,
G. Mustoslavsky, A. C. Serre, E. Y. Snyder, J. J. Yoo, M. E. Furth, S.
Soker, and A. Atala. 2007. Isolation of amniotic stem cell lines with
potential for therapy. Nat. Biotechnol. 25: 100–106.
Ciavarella, S., F. Dammacco, M. De Matteo, G. Loverro, and F.
Silvestris. 2009. Umbilical cord mesenchymal stem cells: role of
regulatory genes in their differentiation to osteoblasts. Stem Cells Dev.
: 1211–1220.
Castillo, M., K. Liu, L. Bonilla, and P. Rameshwar. 2007. The immune
properties of mesenchymal stem cells. Int. J. Biomed. Sci. 3: 76–80.
Mariotti, V., S. J. Greco, R. D. Mohan, G. R. Nahas, and P. Rameshwar.
Stem cell in alternative treatment for brain tumors: potential for
gene delivery. Mol. and Cell. Ther. 2: 1–10.
Pendleton, C., Q. Li, D. A. Chesler, K. Yuan, H. Guerrero-Cazares, and
A. Quinones-Hinojosa. 2013. Mesenchymal stem cells derived from
adipose tissue vs bone marrow: in vitro comparison of their tropism
towards gliomas. PLos One 8:e58198.
Lamfers, M., S. Idema, F. van Milligen, T. Schouten, P. van der Valk,
P. Vandertop, C. Dirven, and D. Noske. 2009. Homing properties of
adipose-derived stem cells to intracerebral glioma and the effects of
adenovirus infection. Cancer Lett. 274: 78–87.
Abdulrazzak, H., D. Moschidou, G. Jones, and P.V. Guillot. 2010.
Biological characteristics of stem cells from foetal, cord blood and
extraembryonic tissues. J. R. Soc. Interface 7:S689–S706.
Kelly, S., T. M. Bliss, A. K. Shah, G. H. Sun, M. Ma, W. C. Foo, J.
Masel, M. A. Yenari, I. L. Weissman, N. Uchida, T. Palmer, and G. K.
Steinberg. 2004. Transplanted human fetal neural stem cells survive,
migrate, and differentiate in ischemic rat cerebral cortex. Proc. Natl.
Acad. Sci. USA 101: 11839–11844.
Jablonska, A., H. Kozlowska, I. Markiewicz, K. Domanska-Janik, and
B. Lukomska. 2010. Transplantation of neural stem cells derived from
human cord blood to the brain of adult and neonatal rats. Acta Neurobiol.
Exp. 70: 337–350.
Armstrong, R. J., C. Watts, C. N. Svendsen, S. B. Dunnett, and A. E.
Rosser. 2000. Survival, neuronal differentiation, and fiber outgrowth
of propagated human neural precursor grafts in an animal model of
Huntington’s disease. Cell Transplant. 9: 55–64.
Lee, A. S., C. Tang, M. S. Rao, I. L. Weissman, and J. C. Wu. 2013.
Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies.
Nat. Med. 19: 998–1004.
Chen, K. G., B. S. Mallon, R. D. G. McKay, and P. G. Robey. 2014.
Human pluripotent stem cell culture: considerations for maintenance,
expansion, and therapeutics. Cell Stem Cell 14: 13–26.
Petit, G. H., T. T. Olsson, and P. Brundin. 2014. The future of cell
therapies and brain repair: Parkinson’s disease. Neuropathol. Appl.
Neurobiol. 40: 60–70.
Park, D. Y., R. E. Mayle, R. L. Smith, I. Corcoran-Schwartz, A.
I. Kharizi, and I. Cheng. 2013. Combined transplantation of human
neuronal and mesenchymal stem cells following spinal cord injury.
Global Spine J. 3: 1–6.
Borlongan, C. V., L. E. Glover, N. Tajiri, Y. Kaneko, and T. B.
Freeman. 2011. The great migration of bone marrow-derived stem cells
toward the ischemic brain: therapeutic implications for stroke and other
neurological disorders. Prog. Neurobiol. 95: 213–228.
Zhao, F., Y. Qu, H. Liu, B. Du, and D. Mu. 2014. Umbilical cord
blood mesenchymal stem cells co-modified by TERT and BDNF: a
neuroprotective therapy for neonatal hypoxic–ischemic brain damage.
Int. J. Dev. Neurosci. 38: 147–154.
Fu, L., L. Zhu, Y. Huang, T. D. Lee, S. J. Forman, C. C. Shih. 2008.
Derivation of neural stem cells from mesenchymal stemcells: evidence
for a bipotential stem cell population. Stem Cells Dev. 17: 1109–1121.
Castaño, J., P. Menendez, C. Bruzos-Cidon, M. Straccia, A. Sousa,
L. Zabaleta, N. Vazquez, A. Zubiarrain, K. C. Sonntag, L. Ugedo, X.
Carvajal-Vergara, J. M. Canals, M. Torrecilla, R. Sanchez-Pernaute,
and A. Giorgetti. 2014. Fast and efficient neural conversion of human
hematopoietic cells. Stem Cell Rep. 3: 1118–1131.
Sun, T., and Q. H. Ma. 2013. Repairing neural injuries using human
umbilical cord blood. Mol. Neurobiol. 47: 938–945.
Sanai, N., A. Alvarez-Buyla, and M. S. Berger. 2005. Neural stem cells
and the origin of gliomas. N Engl. J. Med. 335: 811–822.
Gage, F. H. 2000. Mammalian neural stem cells. Science 287:
–1438.
Doetsch, F., I. Caille, D. A. Lim, J.M. Garcia-Verdugo, and A. Alvarez-
Buylla. 1999. Subventricular zone astrocytes are neural stem cells in
the adult mammalian brain. Cell 97: 703–716.
Sohur, U. S., J. G. Emsley, B. D. Mitchell, and J. D. Macklis. 2006.
Adult neurogenesis and cellular brain repair with neural progenitors,
precursors, and stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci.
: 1477–1497.
Emsley, J. G., B. D. Mitchell, G. Kempermann, and J. D. Macklis. 2005.
Adult neurogenesis and repair of the adult CNS with neural progenitors,
precursors, and stem cells. Prog. Neurobiol. 75: 321–341.
Galvin, K. A. and D. G. Jones. 2006. Adult human neural stem cells for
autologous cell replacement therapies for neurodegenerative disorders.
NeuroRehabilitation 21: 255–265.
Arsenijevic, Y., J. G. Villemure, J. F. Brunet, J. J. Bloch, N. Deglon, C.
Kostic, A. Zurn, and P. Aebischer. 2001. Isolation of multipotent neural
precursors residing in the cortex of the adult human brain. Exp. Neurol.
: 48–62.
Kukekov, V.G., E. D. Laywell, O. Suslov, K. Davies, B. Scheffler, L. B.
Thomas, T. F. O’Brien, M. Kusakabe, and D. A. Steindler. Multipotent
stem/progenitor cells with similar properties arise from two neurogenic
regions of adult human brain. Exp. Neurol. 156: 333–344.
Juengst, E., and M. Fossel. 2000. The ethics of embryonic stem cells—
now and forever, cells without end. J. Am. Med. A 284: 3180–3184.
Kornblum, H. I. 2007. Introduction to neural stem cells. Stroke 38:
–816.
Fuentealba, L. C., K. Obernier, and A. Alvarez-Buylla. 2012. Adult
neural stem cells bridge their niche. Cell Stem Cell 10: 698–708.
Chiu, A. Y., and M. S. Rao. 2011. Cell-based therapy for neural
disorders-anticipating challenges. Neurotherapeutics 8 (4): 744–752.
Lindvall, O., and Z. Kokaia. 2010. Stem cells in human neurodegen-
erative disorders–time for clinical translation? J. Clin. Invest. 120 (1):
–40.
De Filippis, L., and E. Binda. Concise review: self-renewal in the central
nervous system: neural stem cells from embryo to adult. Stem Cells
Transl. Med. 1 (4): 298–308.
Dantuma, E., S. Merchant, K. Sugaya. 2010. Stem cells in human
neurodegenerative disorders-time for clinical translation? J Clin. Invest.
(5): 37.
Dubois, L G., L. Campanati, C. Righy, I. D’Andrea-Meira, T. C. Leite de
Sampaio e Spohr, I. Porto-Carreiro, C. M. Pereira, J. Balca-Silva, S. A.
Kahn, M. F. DosSantos, M. De Almeida Rabello Oliveira, A. Ximenes-
da Silva, M. C. Lopes, E. Faveret, E. Leandro, and G. V. Moura-Neto.
Gliomas and the vascular fragility of the blood brain barrier.
Front. Cell Neurosci. 8: 418.
Lossinsky, A. S., and R.R. Schivers. 2004. Structural pathways for
macromolecular and cellular transport across the blood–brain barrier
during inflammatory conditions. Review. Histol. Histopathol. 19 (2):
–564.
Aleynik, A., K. M. Gernavage, Y. S. H. Mourad, L. S. Sherman, K. Liu,
Y. A. Gubenko, and P. Rameshwar. 2014. Stem cell delivery of therapies
for brain disorders. Clin. Transl. Med. 3: 24 doi:10.1186/2001-1326-3-
Pardridge, W. M. 2006. Molecular Trojan horses for blood–brain barrier
drug delivery. Discov. Med. 6 (5): 494–500.
Pardridge, W., M. 2001. Brain Drug targeting and gene technologies.
Jpn. J. Pharmacol. 87 (2): 97–103.
Pardridge W. M. 2000. Blood–brain barrier drug targeting enables neu-
roprotection in brain ischemia following delayed intravenous admin-
istration of neurotrophins. In: Madame Curie Bioscience Database
[Internet]. Landes Bioscience 2000, Austin, TX. Available from:
http://www.ncbi.nlm.nih.gov/books/NBK5974/
Aiken, R. 2014. Molecular neuro-oncology and the challenge of the
blood–brain barrier. Semin. Oncol. 41 (4): 438–445.
Seelig, A., R. Gottschlich, and R. M. Devant. 1994. A method to
determine the ability of drugs to diffuse through the blood–brain barrier.
PNAS 91: 68–72.
Boado, R. J., E. Ka-Wai Hui, J. Zhiqiang Lu, and W. M. Pardridge.
Insulin receptor antibody–iduronate 2-sulfatase fusion protein:
pharmacokinetics, anti-drug antibody, and safety pharmacology in
rhesus monkeys. Biotechnol. Bioeng. 111 (11): 2317–25.
Reagan, M. R., and D. L. Kaplan. 2011. Concise review: mesenchymal
stem cell tumor-homing: detection methods in disease model systems.
Stem Cells 29 (6): 920–927.
Jordan, C. T., M. L. Guzman, and M. Noble. 2006. Cancer stem cells.
N Eng. J. Med. 335: 1253–1261.
Xiangpen, Y., J. Curtis, X. Yizhi, L. Gentao, S. Waschsmann-Hogiu, D.
L. Farkas, K. L. Black, and J. S. Yu. 2004. Isolation of cancer stem cells
from adult glioblastoma multiforme. Oncogene 23 (58): 9392–9400.
Alieva, M., J. R. Bago, E.Aguilar, C. Soler-Botija, O. F. Vila, J. Molet, S.
S. Gambhir, N. Rubio, and J. Blanco. 2012. Glioblastoma therapy with cytotoxic mesenchymal stromal cells optimized by bioluminescence
imaging of tumor and therapeutic cell response. PloS One 7 (4):e35148.
Altanerova, V., M. Cihova, M. Babic, B. Rychly, K. Ondicova,
B. Mravec, and C. Altaner. 2012. Human adipose tissue-derived
mesenchymal stem cells expressing yeast cytosinedeaminase::uracil
phosphoribosyltransferase inhibit intracerebral rat glioblastoma. Int. J.
Cancer 130 (10): 2455–2463.
Roger, M., A. Clavreul, N. Trinh Huynh, C. Passirani, P. Schiller, A.
Vessi`eres, C. Montero-Meneia, and P. Menei. 2011. Ferrociphenol lipid
nanocapsule delivery by mesenchymal stromal cells in brain tumor
therapy. Int. J. Pharm. 423 (1): 63–68.
Kim, S. M., J. S. Woo, C. H. Jeong, C. H. Ryu, J. D. Jang, and S.
S. Jeun. 2014. Potential application of temozolomide in mesenchymal
stem cell-based trail gene therapy against malignant glioma. Stem Cells
Transl. Med. 3 (2): 172–182.
Snyder, E. Y., X. O. Breakefield, K. S. Aboody, U. Herrlinger, and W.
P. Lynch, inventor The Children’s MedicalCenter Corporation Boston,
MA, USA; The General Hospital Corporation, Charlestown, MA, USA;
Northeastern Ohio Universities College of Medicine, Rootstown, OH,
USA assignee. US Patent # 7186409. Neural stem cells and use
thereof for brain tumor therapy. United States 2007.
Ahmed, A. U., B. Thaci, A. L. Tobias, B. Auffinger, L. Zhang, Y. Cheng,
C. K. Kim, C. Yunis, Y. Han, N. G. Alexiades, X. Fan, K. S. Aboody,
and M. S. Lesniak. 2012. A preclinical evaluation of neural stem cell-
based cell carrier for targeted antiglioma oncolytic virotherapy. JNCI
(13): 968–977.
Cheng, Y., R. Morshed, S. H. Cheng, A. Tobias, B. Auffinger, D. A.
Wainwright, L. Zhang, C. Yunis, Y. Han, C. T. Chen, L. W. Lo, K.
S. Aboody, A. U. Ahmed, and M. S. Lesniak. 2013. Nanoparticle-
programmed self-destructive neural stem cells for glioblastoma target-
ing and therapy. Small 9 (24): 4123–4129.
Larsen, J. M., D. R. Martin., and M. E. Byrne. 2014. Recent advances
in delivery through the blood–brain barrier. Curr. Top. Med. Chem. 14
(9): 1148–1160.
Brem, H., M. G. Ewend, S. Piantadosi, J. Greenhoot, P. C. Burger, and
M. Sisti. 1995. The safety of interstitial chemotherapy with BCNU-
loaded polymer followed by radiation therapy in the treatment of newly
diagnosed malignant gliomas: phase I trial. J. Neurooncol. 26: 111–123.
Hart, M. G., R. Grant, R. Garside, G. Rogers, M. Somerville, and K.
Stein. 2011. Chemotherapy wafers for high grade glioma. Cochrane
Database Syst. Rev. 3. doi:10.1002/14651858.CD007294.pub2.
Chowdhary, S. A., T. Ryken, and H. B. Newton. 2015. Survival out-
comes and safety of carmustine wafers in the treatment of high-grade
gliomas: a meta-analysis. J. Neurooncol. 122 (2): 367–382.
Salahuddin, T. S., B. B. Johansson, H. Kalimo, and Y. Olsson. 1988.
Structural changes in the rat brain after carotid infusions of hyperos-
molar solutions: a light microscopic and immunohistochemical study.
Neuropathol. Appl. Neurobiol. 77: 5–13.
Burgess, A., and K. Hynynen. 2013. Noninvasive and targeted drug
delivery to the brain using focused ultrasound ACS Chem. Neurosci. 4
(4): 519–526.
Egleton, R. D., and T. P. Davis. 2005. Development of neuropeptide
drugs that cross the blood–brain barrier. Neurotherapeutics 2 (1):
–53.
Ramalho-Santos, M., S. Yoon, Y. Matsuzaki, R. C. Mulligan, and D. A.
Melton. 2002. “Stemness”: transcriptional profiling of embryonic and
adult stem cells. Science 298 (5593): 597–600.
Sun, J., A. Ramos, B. Chapman, J. B. Johnnidis, L. Le, Y. J. Ho, A.
Klein, O. Hofmann, and F. D. Camargo. 2014. Clonal dynamics of
native haematopoiesis. Nature 514 (7522): 322–327.
Ning, J., and H. Wakimoto. 2014. Oncolytic herpes simplex virus-
based strategies: toward a breakthrough in glioblastoma therapy. Front.
Microbiol. 5 (303): 1–13.
Kaufmann, J. K., and E. A. Chiocca. 2014. Glioma virus therapies
between bench and bedside. Neuro-Oncology 16 (3): 334–351.
Kaufmann, J. K., and E.A. 2014. Chiocca. Glioma virus therapies
between bench and bedside. Neuro-Oncology 16: 334–351.
Russell, S. J., K. W. Peng, and J. C. Bell. 2014. Oncolytic virotherapy.
Nat. Biotechnol. 30: 1–29.
Wollmann, G., K. Ozduman, and A. N. van den Pol. 2012. Oncolytic
virus therapy of glioblastoma multiforme – concepts and candidates.
Cancer J. 18 (1): 69–81.
Rubsam, L. Z., P. D. Boucher, P. J. Murphy, M. KuKuruga, and
D. S. Shewach. 1999. Cytotoxicity and accumulation of ganciclovir
triphosphate in bystander cells cocultured with herpes simplex virus
type 1 thymidine kinase-expressing human glioblastoma cells. Cancer
Res. 59: 669–675.
Beck, C., S. Cayeux, S. D. Lupton, B. Dörken, and T. Blankenstein.
The thymidine kinase/ganciclovir-mediated “suicide” effect is
variable in different tumor cells. Hum. Gene Ther. 6: 1525–1530.
Colombo, F., L. Barzon, E. Franchin, M. Pacenti, V. Pinna, D. Danieli,
M. Zanusso, and G. Palù. 2005. Combined HSV-TK/IL-2 gene therapy
in patients with recurrent glioblastoma multiforme: biological and
clinical results. Cancer Gene Ther. 12: 835–848.
Perry, J. R. 2012. Thromboembolic disease in patients with high-grade
glioma. Neuro-Oncology 14 (Suppl. iv): iv73–iv80.
De Cicco, M. 2004. The prothrombotic state in cancer: pathogenic
mechanisms. Crit. Rev. Oncol. Hematol. 50 (3): 187–196.
McKie, E. A., A. R. MacLean, A.D. Lewis, G. Cruickshank, R. Ram-
pling, S.C. Barnett, P.G. Kennedy, S.M. Brown. 1996. Selective in vitro
replication of herpes simplex virus type 1 (HSV-1) ICP34.5 null mutants
in primary human CNS tumours—evaluation of a potentially effective
clinical therapy. Br J Cancer 74 (5): 745–752.
Mineta, T., S. D. Rabkin, T. Yazaki, W. D. Hunter, R. L. Martuza. 1995.
Attentuated multi-mutated herpes simplex virus-1 for the treatment of
malignant gliomas. Nat. Med. 1 (9): 938–943.
Freeman, A. I., Z. Zakay-Rones, J. M. Gomori, E. Linetsky, L. Rasooly,
E. Greenbaum, S. Rozenman-Yair, A. Panet, E. Libson, C.S. Irving, E.
Galun, and T. Seigal. 2006. Phase I/II trial of intravenous NDV-HUJ
oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 13:
–228.
Rainov, N.G. 2000. A Phase III Clinical evaluation of herpes sim-
plex virus type 1 thymidine kinase and ganciclovir gene therapy as
an adjuvant to surgical resection and radiation in adults with previ-
ously untreated glioblastoma multiforme. Hum. Gene Ther. 11 (17):
–2401.
Yla-Herttuala, M. W. S., J. Martin, P. Warnke, P.Menei, D. Eckland, J.
Kinley, R. Kay, and Z. Ram. 2013. Adenovirus-mediated gene therapy
with sitimagene ceradenovec followed by intravenous ganciclovir for
patients with operable high-grade gliomas (ASPECT): a randomised,
open-label, phase 3 trial. Lancet Oncol. 14 (9): 822–833.
Stupp, R., W. P. Mason, M. J. van den Bent, M. Weller, B. Fisher,
M.J.B. Taphoorn, K. Belanger, A. A. Brandes, C. Marosi, U. Bogdahn,
J. Curschmann, R. C. Janzer, S. K. Ludwin, T. Gorlia, A. Allgeier, D.
Lacombe, J. Gregory Cairncross, E. Eisenhauer, and R. O. Mirimanoff.
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Eng. J. Med. 352: 987–996.
Barnett, S. C., L. Robertson, D. Graham, D. Allan, and R. Rampling.
Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells trans-
formed with c-myc and H-ras form high-grade glioma after stereotactic
injection into the rat brain. Carcinogenesis 19 (9): 1529–1537.
O’Neill, D. W., S. Adams, and N. Bhardwaj. 2004. Manipulating
dendritic cell biology for the active immunotherapy of cancer. Blood
(8): 2235–2246.
Ahmed, M. S., and Y. S. Bae. 2014. Dendritic cell-based therapeutic
cancer vaccines: past, present, and future. Clin. Exp. Vaccine 3 (2):
–116.
Schreiber R. D., L. J. Old, and M. J. Smyth. 2011. Cancer Immunoedit-
ing: Integrating immunity’s roles in cancer suppression and promotion.
Science 331 (6024): 1565–1570.
Fonteneau, J. F., M. Gilliet, M. Larsson, I. Dasilva, C. Munz, Y. J. Liu,
and N. Bhardwaj. 2003.Activation of influenza virus-specific CD4+ and
CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive
immunity. Blood 101 (9): 3520–3526.
Salio, M., M. Cella, W. Vermi, F. Facchetti, M. J. Palmowski, C. L.
Smith, D. Shepherd, M. Colonna, and V. Cerundulo. 2003. Plasmacytoid
dendritic cells prime IFN-gamma-secreting melanoma-specific CD8
lymphocytes and are found in primary melanoma lesions. Eur. J.
Immunol. 33: 1052–1062.
Eshhar, Z., T. Waks, G. Cross, and D. G. Schindler. 1993. Specific
activation and targeting of cytotoxiclymphocytes through chimeric
single chains consisting of antibody-binding domains and the y or
C subunits of the immunoglobulinand T-cell receptors. PNAS 90:
–724.
Miao, H., B. D. Choi, C. M. Suryadevara, L. Sanchez-Perez, S. Yang,
G. De Leon, E. J. Sayor, R. McLendon, J. E. Herndon II, P. Healy,
G. E. Archer, D. D. Binger, L. A. Johnson, and J. H. Sampson. 2014.
EGFRvIII-specific chimeric antigen receptor T cells migrate to and
kill tumor deposits infiltrating the brain parenchyma in an invasive
xenograft model of glioblastoma. PLos One 9 (4): 1–9.
Lee, D.W., D. M. Barrett, C. Mackall, R. Orentas, and S. A. Grupp.
The future is now: chimeric antigen receptors as new targeted
therapies for childhood cancer. CCR Focus 18 (10): 2780–2790.
Imai, C., K. Mihara, M. Andreansky, I. C. Nicholson, C. H. Pui, T. L.
Geiger, and D. Campana. 2004. Chimeric receptors with 4-1BB signal-
ing capacity provoke potent cytotoxicity against acute lymphoblastic
leukemia. Leukemia 18: 676–684.
Carpentino, C., M. C. Milone, R. Hassan, J. C. Simonet, M. Lakhal,
M. M. Suhoski, A. Varela-Rohena, K. M. Haines, D. F. Heitjan, S.
M. Albelda, R. G. Carroll, J. L. Riley, I. Pastan, and C. H. June.
Control of large, established tumor xenografts with genetically
retargeted human T cells containing CD28 and CD137 domains. PNAS
(9): 3360–3365.
Zhao, Y., Q. J. Wang, S. Yang, J. N. Kochenderfer, Z. Zheng, X. Zhong,
M. Sadelain, Z. Eshhar, S. A. Rosenberg, and R. A. Morgan. 2009.
A herceptin-based chimeric antigen receptor with modified signaling
domains leads to enhanced survival of transduced T lymphocytes and
antitumor activity. J. Immunol. 183 (9): 5563–5574.
Morgan, R. A. 2013. Risky business: target choice in adoptive cell
therapy. Blood 122 (20): 3392–3394.
Brentjens, R., R. Yeh, Y. Bernal, I. Riviere, and M. Sadelain. 2010.
Treatment of chronic lymphocytic leukemia with genetically targeted
autologous T cells: case report of an unforeseen adverse event in a phase
I clinical trial. Mol. Ther. 18 (4): 666–668.
Kochenderfer, J. N., M. E. Dudley, S. A. Feldman, W. H. Wilson, D.
E. Spaner, I. Maric, M. Stetler-Stevenson, G. Q. Phan, M. S. Hughes,
R. M. Sherry, J. C. Yang, U. S. Kammula, L. Devillier, R. Carpenter,
D. A. Nathan, R. A. Morgan, C. Laurencot, and S. A. Rosenberg. 2012.
B-cell depletion and remissions of malignancy along with cytokine-
associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-
receptor-transduced T cells. Blood 119 (12): 2709–2720.
Grupp, S. A., M. Kalos, D. Barrett, R. Aplenc, D. L. Porter, S. R.
Rheingold, D. T. Teachey, A. Chew, B. Hauck, J. Fraser Wright, M. C.
Milone, B. L. Levine, and C. H. June. 2013. chimeric antigen receptor-
modified t cells for acute lymphoid leukemia. N Engl. J. Med. 368 (16):
–1518.
Le Huu, D., T. Matsushita, G. Jin, Y. Hamaguchi, M. Hasegawa, K.
Takehara, and M. Fujimoto. 2012. IL-6 blockade attenuates the devel-
opment of murine sclerodermatous chronic graft-versus-host disease.
J. Invest. Dermatol. 132: 2752–2761.
Pule, M. A., B. Savoldo, G. D. Myers, C. Rossig, H. V. Russell, G. Dotti,
M. H. Huls, E. Liu, A. P. Gee, Z. Mei, E. Yvon, H. L. Weiss, H. Liu,
C. M. Rooney, H. E. Heslop, and M. K. Brenner. 2008. Virus-specific
T cells engineered to coexpress tumor-specific receptors: persistence
and antitumor activity in individuals with neuroblastoma. Nat. Med. 14
(11): 1264–1270.
Hatiboglu, M. A., J. Wei, A. S. G. Wu, and A. B. Heimberger. 2010.
Immune therapeutic targeting of glioma cancer stem cells. Target Oncol.
(3): 217–227.
Barkholt, L., E. Flory, V. Jekerle, S. Lucas-Samuel, P. Ahnert, L. Bisset,
D. Büscher, W. Fibbe, A. Foussat, M. Kwa, O. Lantz, R. Maˇciulaitis,
T. Palomäki, C. K. Schneider, L. Sensebé, G. Tachdjian, K. Tarte, L.
Tosca, and P. Salmikangas. 2013. Risk of tumorigenicity in mesenchy-
mal stromal cell-based therapies–bridging scientific observations and
regulatory viewpoints. Cytotherapy 15 (7): 753–759.
Harsh, G. R., T. S. Deisboeck, D. N. Loius, J. Hilton, M. Colvin, J. S.
Silver, N. H. Qureshi, J. Kracher, D. Finkelstein, E. A. Chiocca, and
F. H. Hochberg. 2000. Thymidine kinase activation of ganciclovir in
recurrent malignant gliomas: a gene-marking and neuropathological
study. J. Neurosurg. 92 (5): 804–811.
Smith, A.G. 2001. Embryo-derived stem cells: of mice and men. Annu.
Rev. Cell Dev. Biol. 17: 435–462.
Le Blanc, K., and M. F. Pittenger. 2005. Mesenchymal stem cells:
progress toward promise. Cytotherapy 7 (1): 36–45.
Tolar, J., A. J. Nauta, M. J. Osborn, A. P. Mortari, R. T. McElmurry, S.
Bell, L. Xia, N. Zhou, M. Riddle, T. M. Schroeder, J. J. Westendorf,
R. S. McIvor, P. C. W. Hogendoorn, K. Szuhai, L. Oseth, B. Hirsch, S.
R. Yant, M. A. Kay, A. Peister, D. J. Prockop, W. E. Fibbe, and B. R.
Blazar. 2007. Sarcoma derived from cultured mesenchymal stem cells.
Stem Cells 25: 371–379.
Atsma D. E., W. E. Fibbe, and T. J. Rabelink. 2007. Opportunities
and challenges for mesenchymal stem cell-mediated heart repair. Curr.
Opin. Lipidol. 18 (6): 645–649.
Solchaga, L. A., K. J. Penick, and J. F. Welter. 2011. Chondrogenic
differentiation of bone marrow-derived mesenchymal stem cells: Tips
and Tricks. Methods Mol. Biol. 698: 253–278.
Wei X., X. Yang, Z. P. Han, F. F. Qu, L. Shao, and Y. F. Shi. 2013.
Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol.
Sinica 34 (6): 747–754.
Ryan, J. M., F. P. Barry, J. M. Murphy, and B. P. Mahon. 2005.
Mesenchymal stem cells avoid allogeneic rejection. J. Inflam. 2: 8.
Medvedev, S. P., A. I. Shevchenk, and S. M. Zakian. 2010. Induced
pluripotent stem cells: problems and advantages when applying them
in regenerative medicine. ACTA NAT. 2 (5): 18–28.
Yamanaka, S. 2009. A FRESH Look at iPS cells. Cell 137 (1): 13–17.
Chan, T. M., J. Y. R. Chen, L. I. Ho, H. P. Lin, K. W. Hsueh, D. D. Liu,
Y. H. Chen, A. C. Hsieh, N. M. Tsai, D. Y. Hueng, S. T. Tsai, P. W. Chou,
S. Z. Lin, and H. J. Harn. 2014. ADSC Therapy in neurodegenerative
disorders. Cell Transplant. 23 (4–5): 549–557.
Desplats P., H. J. Lee, E. J. Bae, C. Patrick, E. Rockenstein, L. Crews,
B. Spencer, E. Masliah, and S. J. Lee. 2009. Inclusion formation and
neuronal cell death through neuron-to-neuron transmission of alpha-
synuclein. PNAS 106 (31): 13010–13015.
Sanberg, P. R., D. J. Eve, A. E. Willing, S. Garbuzova-Davis, J. Tan, C.
D. Sanberg, J. G. Allickson, E. L. Cruz, and C. V. Borlongan. 2011.
The treatment of neurodegenerative disorders using umbilical cord
blood and menstrual blood-derived stem cells. Cell Transplant. 20 (10):
–94.
Spinelli V., P. V. Guillot, and P. De Coppi. 2013. Induced pluripotent
stem (iPS) cells from human fetal stem cells (hFSCs). Organogenesis
(2): 101–110.
Broxmeyer, H. E. 2010. Umbilical cord transplantation: Epilogue.
Semin. Hematol. 47 (1): 97–103.
Altaner, C., V. Altanerova, M. Cihova, K. Ondicova, B. Rychly, L.
Baciak, and B. Mravec. 2014. Complete regression of glioblastoma
by mesenchymal stem cells mediated prodrug gene therapy simulating
clinical therapeutic scenario. International J. Cancer 134 (6): 1458–
Egleton, R. D., and T. P. Davis. 2005. Development of neuropeptide
drugs that cross the blood–brain barrier. NeuroRx 2 (1): 44–53.
Qin, J., X. Yang, J. Mi, J. Wang, J. Hou, T. Shen, Y. Li, B. Wang,
X. Li, and W. Zhu. 2014. Enhanced antidepressant-like effects of the
macromolecule trefoil factor 3 by loading into negatively charged
liposomes. Int. J. Nanomed. 9: 5247–5257.
Wang, X., P. Liu, W. Yang, L. Li, P. Li, Z. Liu, Z. Zhuo, and Y.
Gao. Microbubbles coupled to methotrexate-loaded liposomes for
ultrasound-mediated delivery of methotrexate across the blood–brain
barrier. Int. J. Med.; 9: 4899–4909.
Steiniger, S. C., J. Kreuter, A. S. Khalansky, I. N. Skidan, A. I.
Bobruskin, Z. S. Smirnova, S. E. Severin, R. Uhl, M. Kock, K. D.
Geiger, and S. E. Gelperina. Chemotherapy of glioblastoma in rats using
doxorubicin-loaded nanoparticles. Int. J. Cancer. 109 (5): 759–767.
Jones, A. R., and E. V. Shusta. 2007. Blood–Brain Barrier Transport of
Therapeutics via Receptor-Mediation. Pharm. Res. 24 (9): 1759–1771.
Pardridge, W. M. 2001. Brain Drug Targeting and Gene Technologies.
Jpn. J. Pharmacol. 87 (2): 97–103.
Wait, S. D., R. S. Prabhu, S. H. Burri, T. G. Atkins, and A. L. Asher.
Polymeric drug delivery for the treatment of glioblastoma. Neuro-
Oncology 17 (2): ii9–ii23.
Larsen, J.M., D. R. Martin, and M. E. Byrne. 2014. Recent advances
in delivery through the blood–brain barrier. Curr. Top. Med. Chem. 14
(9): 1148–1160.
Jewell, C.M., S. C. Bustamante Lopez, and D. J. Irvine. 2011. In
situ engineering of the lymph node microenvironment via intranodal
injection of adjuvant-releasing polymer particles. PNAS 108 (38):
–15750.
Burgess, A., C.A. Ayala-Grosso, M. Ganguly, J.F. Jordão, I. Aubert, K.
Hynynen. 2011. Targeted Delivery of Neural Stem Cells to the Brain
Using MRI-Guided Focused Ultrasound to Disrupt the Blood–Brain
Barrier. PLoS ONE 6 (11): e27877.
Balyasnikova, I. V., M. S. Prasol, S. D. Ferguson, Y. Han, A. U. Ahmed,
M. Gutova,A. L. Tobias, D. Mustafi, E. Rincon, L. Zhang, K. S.Aboody,
and M. S. Lesniak. 2014. Intranasal delivery of mesenchymal stem cells
significantly extends survival of irradiated mice with experimental brain
tumors. Mol. Ther. 22: 140–148.
Sampson, J. H., K. D. Alpade, G. E. Archer, A. Coan, A. Desjardins,
A. H. Friedman, H. S. Friedman, M. R. Gilbert, J. E. Herndon, R. E.
McLendon, D. A. Mitchell, D. A. Reardon, R. Sawaya, R. Schmittling,
W. Shi, J. J. Vredenburgh, D. D. Bigner, and A. B. Heimberger. 2011.
Greater chemotherapy-induced lymphopenia enhances tumor-specific
immune responses that eliminate EGFRvIII-expressing tumor cells in
patients with glioblastoma. Neuro-Oncology 13 (3): 324–333.
Phuphanich, S., C. J. Wheeler, J. D. Rudnick, M. Mazer, H. Q. Wang,
M. A. Nuno, J. E. Richardson, X. Fan, J. Ji, R.M. Chu, J. G. Bender, E.
S. Hawkins, C. G. Patil, K. L. Black, and J. S. Yu. 2013. Phase I trial
of a multi-epitope-pulsed dendritic cell vaccine for patients with newly
diagnosed glioblastoma. Cancer Immunol. Immunother. 62: 125–135.
Fadul, C. E., J. L. Fisher, T. H. Hampton, E. C. Lallana, Z. Li, J.
Gui, Z. M. Szczepiorkowki, T. D. Tosteson, C. H. Rhodes, H. A.
Wishart, L. D. Lewis, and M. S. Ernstoff. 2011. Immune response
in patients with newly diagnosed glioblastoma multiforme treated
with intranodal autologous tumor lysate-dendritic cell vaccination after
radiation chemotherapy. J. Immunother. 34 (4): 382–389.
Shand, N., F. Weber, L. Mariani, M. Bernstein, A. Gianella-Borradi,
Z. Long, A. G. Sorensen, and N. Barbier. 1999. A phase 1–2 clinical
trial of gene therapy for recurrent glioblastoma multiforme by tumor
transduction with the herpes simplex thymidine kinase gene followed
by ganciclovir. Hum. Gene Ther. 10 (14): 2325–2335.
Markert, J. M., P. G. Liechty, W. Wang, S. Gaston, E. Braz, M. Karrasch,
L. B. Nabors, M. Markiewicz, A. D. Lakeman, C. A. Palmer, J. N.
Parker, R. J. Whitley, G. Y. Gillespie. 2009. Phase Ib trial of mutant
herpes simplex virus G207 inoculated pre-and post-tumor resection for
recurrent GBM. Mol. Ther. 17 (1): 199–207.